Appel reaction
Encyclopedia
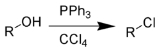
The Appel reaction is an organic reaction
that converts an alcohol
into an alkyl chloride using triphenylphosphine
and carbon tetrachloride
. The use of carbon tetrabromide or bromine
as a halide source will yield alkyl bromides, whereas using methyl iodide or iodine
gives alkyl iodides. The reaction is credited to and named after Rolf Appel
, it had however been described earlier.
 Drawbacks to the reaction are the use of toxic halogenating agents and the coproduction of organophosphorus product that must be separated from the organic product. The phosphorus reagent can be used in catalytic
Drawbacks to the reaction are the use of toxic halogenating agents and the coproduction of organophosphorus product that must be separated from the organic product. The phosphorus reagent can be used in catalytic
quantities. The corresponding alkyl bromide can also be synthesised by addition of lithium bromide
as a source of bromide ions.
salt 2. Deprotonation
of the alcohol, forming chloroform
3, yields an alkoxide ion pair 4. The nucleophilic displacement of the chloride by the alkoxide yields intermediate 5. With primary and secondary alcohols, the halide reacts in a SN2
process forming the alkyl halide 6 and triphenylphosphine oxide
7. Tertiary alcohols form the products 6 and 7 via a SN1
mechanism.
The driving force behind this and similar reactions is provided by the formation of triphenylphosphine oxide. The reaction is somewhat similar to the Mitsunobu Reaction
, where the combination of an organophosphine as an oxide acceptor, a diazo compound as a hydrogen acceptor reagent, and a nucleophile
are used to convert alcohols to esters and other applications like this.
 Illustrative use of the Appel reaction is the chlorination of geraniol
Illustrative use of the Appel reaction is the chlorination of geraniol
to geranyl chloride.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
that converts an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
into an alkyl chloride using triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
and carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
. The use of carbon tetrabromide or bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
as a halide source will yield alkyl bromides, whereas using methyl iodide or iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
gives alkyl iodides. The reaction is credited to and named after Rolf Appel
Rolf Appel
Rolf Appel was an inorganic chemist who worked in the area of organophosphorus chemistry. Appel was born in 1921.-Education:He received his PhD at age 30. Appel was appointed in 1962 to both the University of Bonn along with the inorganic chemical institute in 1962 from the University of Heidelberg...
, it had however been described earlier.

Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
quantities. The corresponding alkyl bromide can also be synthesised by addition of lithium bromide
Lithium bromide
Lithium bromide, or LiBr, is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems.-Production and properties:...
as a source of bromide ions.
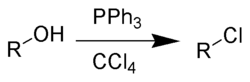
Mechanism
The Appel reaction begins with the formation of the phosphoniumPhosphonium
The phosphonium cation describes positively charged polyatomic cations with the chemical formula . Salts of the parent PH4+ are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakisphosphonium chloride:Organic phosphonium salts are common...
salt 2. Deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of the alcohol, forming chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
3, yields an alkoxide ion pair 4. The nucleophilic displacement of the chloride by the alkoxide yields intermediate 5. With primary and secondary alcohols, the halide reacts in a SN2
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
process forming the alkyl halide 6 and triphenylphosphine oxide
Triphenylphosphine oxide
Triphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
7. Tertiary alcohols form the products 6 and 7 via a SN1
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
mechanism.
The driving force behind this and similar reactions is provided by the formation of triphenylphosphine oxide. The reaction is somewhat similar to the Mitsunobu Reaction
Mitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate or diisopropyl azodicarboxylate . The alcohol undergoes an inversion of stereochemistry...
, where the combination of an organophosphine as an oxide acceptor, a diazo compound as a hydrogen acceptor reagent, and a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
are used to convert alcohols to esters and other applications like this.

Geraniol
Geraniol is a monoterpenoid and an alcohol. It is the primary part of rose oil, palmarosa oil, and citronella oil . It also occurs in small quantities in geranium, lemon, and many other essential oils. It appears as a clear to pale-yellow oil that is insoluble in water, but soluble in most common...
to geranyl chloride.

