Organochloride
Encyclopedia
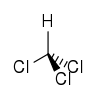 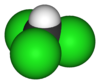 |
| Two representations of the organochloride chloroform Chloroform Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous... . |
An organochloride, organochlorine, chlorocarbon, chlorinated hydrocarbon, or chlorinated solvent is an organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
containing at least one covalently bonded
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
atom. Their wide structural variety and divergent chemical properties lead to a broad range of applications. Many derivatives are controversial because of the effects of these compounds on the environment and on human and animal health.
Physical properties
Chloride substituents modify the physical properties of organic compounds in several ways. They are typically denser than waterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
due to the presence of high atomic weight of chlorine. Chloride substituents induce stronger intermolecular interactions than hydrogen substituents. The effect is illustrated by trends in boiling points: methane (-161.6 °C), methyl chloride (-24.2 °C), dichloromethane (40 °C), chloroform (61.2 °C), and carbon tetrachloride (76.72 °C). The increased intermolecular interactions is attributed to the effects of both van der Waals and polarity.
Natural occurrence
Although rare compared to non-halogenated organic compounds, many organochlorine compounds have been isolated from natural sources ranging from bacteria to humans. Chlorinated organic compounds are found in nearly every class of biomolecules including alkaloidAlkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
s, terpene
Terpene
Terpenes are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, though also by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeterium. They are often strong smelling and thus may have had a protective...
s, amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, flavonoid
Flavonoid
Flavonoids , are a class of plant secondary metabolites....
s, steroid
Steroid
A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.The core...
s, and fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s. Organochlorides, including dioxins, are produced in the high temperature environment of forest fires, and dioxins have been found in the preserved ashes of lightning-ignited fires that predate synthetic dioxins. In addition, a variety of simple chlorinated hydrocarbons including dichloromethane, chloroform, and carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
have been isolated from marine algae. A majority of the chloromethane
Chloromethane
Chloromethane, also called methyl chloride, R-40 or HCC 40, is a chemical compound of the group of organic compounds called haloalkanes. It was once widely used as a refrigerant. It is a colorless extremely flammable gas with a minorly sweet odor, which is, however, detected at possibly toxic levels...
in the environment is produced naturally by biological decomposition, forest fires, and volcanoes. The natural organochloride epibatidine
Epibatidine
Epibatidine is an alkaloid found on the skin of the endangered Ecuadorian frog, Epipedobates tricolor. These frogs, like other poison dart frogs, are best known for their ability to sequester poisons from their prey and secrete these poisons onto their backs. Many Amerindian tribes would swipe the...
, an alkaloid isolated from tree frogs, has potent analgesic
Analgesic
An analgesic is any member of the group of drugs used to relieve pain . The word analgesic derives from Greek an- and algos ....
effects and has stimulated research into new pain medication.
From chlorine
Alkanes and arylalkanes may be chlorinated under free radical conditions, with UV light. However, the extent of chlorination is difficult to control. Aryl chlorides may be prepared by the Friedel-Crafts halogenation, using chlorine and a Lewis acidLewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
catalyst.
The haloform reaction
Haloform reaction
The haloform reaction is a chemical reaction where a haloform is produced by the exhaustive halogenation of a methyl ketone in the presence of a base. R may be , alkyl or aryl...
, using chlorine and sodium hydroxide, is also able to generate alkyl halides from methyl ketones, and related compounds. Chloroform was formerly produced thus.
Chlorine adds to the multiple bonds on alkenes and alkynes as well, giving di- or tetra-chloro compounds.
Reaction with hydrogen chloride
Alkenes react with hydrogen chlorideHydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
(HCl) to give alkyl chlorides. For example, the industrial production of chloroethane
Chloroethane
Chloroethane or monochloroethane, commonly known by its old name ethyl chloride, is a chemical compound with chemical formula , once widely used in producing tetraethyllead, a gasoline additive...
proceeds by the reaction of ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
with HCl:
- H2C=CH2 + HCl → CH3CH2Cl
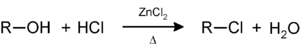
Secondary and tertiary alcohols react with the Lucas reagent (zinc chloride
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
in concentrated hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
) to give the corresponding alkyl halide; this reaction a method for classifying alcohols:
Other chlorinating agents
In the laboratory, alkyl chlorides are most easily prepared by reacting alcohols with thionyl chlorideThionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
(SOCl2), phosphorus trichloride
Phosphorus trichloride
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having chemical formula PCl3. Its shape is trigonal pyramidal. It is the most important of the three phosphorus chlorides. It is an important industrial chemical, being used for the manufacture of organophosphorus compounds...
(PCl3), or phosphorus pentachloride (PCl5):
- ROH + SOCl2 → RCl + SO2 + HCl
- 3 ROH + PCl3 → 3 RCl + H3PO3
- ROH + PCl5 → RCl + POCl3
In the laboratory, thionyl chloride is especially convenient, because the byproducts are gaseous.
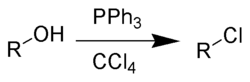
Alternatively, the Appel reaction
Appel reaction
The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using methyl iodide or iodine gives alkyl iodides...
:
Reactions
Alkyl chlorides are versatile building blocks in organic chemistry. While alkyl bromides and iodides are more reactive, alkyl chlorides tend to be less expensive and more readily available. Alkyl chlorides readily undergo attack by nucleophiles.Heating alkyl halides with sodium hydroxide or water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
gives alcohols. Reaction with alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
s or aroxides give ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s in the Williamson ether synthesis
Williamson ether synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and an alcohol. This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction...
; reaction with thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
s give thioether
Thioether
A thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
s. Alkyl chlorides readily react with amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s to give substituted amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. Alkyl chlorides are substituted by softer halides such as the iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
in the Finkelstein reaction
Finkelstein reaction
The Finkelstein reaction, named for the German chemist Hans Finkelstein , is an SN2 reaction that involves the exchange of one halogen atom for another...
. Reaction with other pseudohalides such as azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
, cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
, and thiocyanate
Thiocyanate
Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates...
are possible as well. In the presence of a strong base, alkyl chlorides undergo dehydrohalogenation to give alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s or alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s.
Alkyl chlorides react with magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
to give Grignard reagents, transforming an electrophilic compound into a nucleophilic compound. The Wurtz reaction
Wurtz reaction
The Wurtz reaction, named after Charles-Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium to form a new carbon-carbon bond:...
reductively couples two alkyl halides to couple with sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
.
Vinyl chloride
The largest application of organochlorine chemistry is the production of vinyl chlorideVinyl chloride
Vinyl chloride is the organochloride with the formula H2C:CHCl. It is also called vinyl chloride monomer, VCM or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride . At ambient pressure and temperature, vinyl chloride...
. The annual production in 1985 was around 13 billion kilograms, almost all of which was converted into polyvinylchloride (PVC).
Chloromethanes
Most low molecular weight chlorinated hydrocarbons such as chloroformChloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
, dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
, dichloroethene
Dichloroethene
Dichloroethene or Dichloroethylene, often abbreviated as DCE, can refer to any one of several isomeric forms of the organochloride with the molecular formula C2H2Cl2:There are three isomers:*1,1-Dichloroethene*1,2-Dichloroethene...
, and trichloroethane
Trichloroethane
Trichloroethane can refer to either of two isomeric chemical compounds:* 1,1,1-Trichloroethane * 1,1,2-Trichloroethane...
are useful solvents. These solvents tend to be relatively non-polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
; they are therefore immiscible with water and effective in cleaning applications such as degreasing
Degreasing
Degreasing often called Defatting, is a term used to describe the removal of fatty acids from an object. In culinary science, the word degreasing refers to various methods which are used to reduce the fat content of a meal. This term can be used to categorize procedures that reduce the fat content...
and dry cleaning
Dry cleaning
Dry cleaning is any cleaning process for clothing and textiles using a chemical solvent other than water. The solvent used is typically tetrachloroethylene , abbreviated "perc" in the industry and "dry-cleaning fluid" by the public...
. Several billion kilograms of chlorinated methanes are produced annually, mainly by chlorination of methane:
- CH4 + x Cl2 → CH4-xClx + x HCl
The most important is dichloromethane, which is mainly used as a solvent. Chloromethane is a precursor to chlorosilane
Chlorosilane
Chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond.-Synthesis:...
s and silicone
Silicone
Silicones are inert, synthetic compounds with a variety of forms and uses. Typically heat-resistant and rubber-like, they are used in sealants, adhesives, lubricants, medical applications , cookware, and insulation....
s. Historically significant, but smaller in scale is chloroform, mainly a precursor to chlorodifluoromethane
Chlorodifluoromethane
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon . This colorless gas is better known as HCFC-22, or R-22. It was once commonly used as a propellant and in air conditioning applications...
(CHClF2) and tetrafluoroethene which is used in the manufacture of Teflon.
Pesticides
Many pesticides contain chlorine. Notable examples include DDTDDT
DDT is one of the most well-known synthetic insecticides. It is a chemical with a long, unique, and controversial history....
, dicofol
Dicofol
Dicofol is an organochlorine pesticide that is chemically related to DDT. Dicofol is a miticide that is very effective against red spider mite....
, heptachlor
Heptachlor
Heptachlor is an organochlorine compound that was used as an insecticide. Usually sold as a white or tan powder, heptachlor is one of the cyclodiene insecticides. In 1962, Rachel Carson's Silent Spring questioned the safety of heptachlor and other chlorinated insecticides. Due to its highly...
, endosulfan
Endosulfan
Endosulfan is an off-patent organochlorine insecticide and acaricide that is being phased out globally. Endosulfan became a highly controversial agrichemical due to its acute toxicity, potential for bioaccumulation, and role as an endocrine disruptor...
, chlordane
Chlordane
Chlordane, or chlordan, is an organochlorine compound that was used as a pesticide. This white solid was sold in the U.S. until 1983 as an insecticide for crops like corn and citrus and on lawns and domestic gardens.-Production and uses:...
, aldrin
Aldrin
Aldrin is an organochlorine insecticide that was widely used until the 1970s, when it was banned in most countries. It is a colourless solid. Before the ban, it was heavily used as a pesticide to treat seed and soil...
, dieldrin
Dieldrin
Dieldrin is a chlorinated hydrocarbon originally produced in 1948 by J. Hyman & Co, Denver, as an insecticide. Dieldrin is closely related to aldrin, which reacts further to form dieldrin. Aldrin is not toxic to insects; it is oxidized in the insect to form dieldrin which is the active compound...
, endrin
Endrin
Endrin is an organochloride that was primarily used as an insecticide. It is a colourless odorless solid, although commercial samples are often off-white. It is also a rodenticide. This compound became infamous as persistent organic pollutant and for this reason is banned in many...
, mirex
Mirex
Mirex is a chlorinated hydrocarbon that was commercialized as an insecticide and later banned because of its impact on the environment. This white crystalline odorless solid is a derivative of cyclopentadiene. It was popularized to control fire ants but by virtue of its chemical robustness and...
, and pentachlorophenol
Pentachlorophenol
Pentachlorophenol is an organochlorine compound used as a pesticide and a disinfectant. First produced in the 1930s, it is marketed under many trade names...
. These can be either hydrophilic or hydrophobic depending on their molecular structure. Many of these agents have been banned in various countries, e.g. mirex, aldrin.
Insulators
Polychlorinated biphenylPolychlorinated biphenyl
Polychlorinated biphenyls are a class of organic compounds with 2 to 10 chlorine atoms attached to biphenyl, which is a molecule composed of two benzene rings. The chemical formula for PCBs is C12H10-xClx...
s (PCBs) were once commonly used electrical insulators and heat transfer agents. Their use has generally been phased out due to health concerns. PCBs were replaced by polybrominated diphenyl ethers (PBDEs), which bring similar toxicity and bioaccumulation concerns.
Toxicity
Some types of organochlorides have significant toxicity to plants or animals, including humans. Dioxins, produced when organic matter is burned in the presence of chlorine, and some insecticides such as DDTDDT
DDT is one of the most well-known synthetic insecticides. It is a chemical with a long, unique, and controversial history....
are persistent organic pollutant
Persistent organic pollutant
thumb|right|275px|State parties to the Stockholm Convention on Persistent Organic PollutantsPersistent organic pollutants are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes...
s which pose dangers when they are released into the environment. For example, DDT, which was widely used to control insects in the mid 20th century, also accumulates in food chains, and causes reproductive problems (i.e., eggshell thinning) in certain bird species.
When chlorinated solvents, such as carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
, are not disposed of properly, they accumulate in groundwater. Some highly reactive organochlorides such as phosgene
Phosgene
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy as a chemical weapon during World War I. It is also a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In low concentrations, its odor resembles...
have even been used as chemical warfare agents.
However, the presence of chlorine in an organic compound does not ensure toxicity. Many organochlorides are safe enough for consumption in foods and medicines. For example, peas and broad beans contain the natural chlorinated plant hormone 4-chloroindole-3-acetic acid
4-Chloroindole-3-acetic acid
4-Chloroindole-3-acetic acid is a natural plant hormone. It is a member of the class of compounds known as auxins and a chlorinated derivative of the more common auxin indole-3-acetic acid . 4-Cl-IAA is found in the seeds of a variety of plants, particularly legumes such as peas and broad beans...
(4-Cl-IAA); and the sweetener sucralose
Sucralose
Sucralose is an artificial sweetener. The majority of ingested sucralose is not broken down by the body and therefore it is non-caloric. In the European Union, it is also known under the E number E955. Sucralose is approximately 600 times as sweet as sucrose , twice as sweet as saccharin, and 3.3...
(Splenda) is widely used in diet products. , there were at least 165 organochlorides approved worldwide for use as pharmaceutical drugs, including the natural antibiotic vancomycin
Vancomycin
Vancomycin INN is a glycopeptide antibiotic used in the prophylaxis and treatment of infections caused by Gram-positive bacteria. It has traditionally been reserved as a drug of "last resort", used only after treatment with other antibiotics had failed, although the emergence of...
, the antihistamine loratadine
Loratadine
Loratadine is a second-generation H1 histamine antagonist drug used to treat allergies. Structurally, it is closely related to tricyclic antidepressants such as imipramine, and distantly related to the atypical antipsychotic quetiapine. It is marketed by Schering-Plough under several trade names...
(Claritin), the antidepressant sertraline
Sertraline
Sertraline hydrochloride is an antidepressant of the selective serotonin reuptake inhibitor class. It was introduced to the market by Pfizer in 1991. Sertraline is primarily used to treat major depression in adult outpatients as well as obsessive–compulsive, panic, and social anxiety disorders in...
(Zoloft), the anti-epileptic lamotrigine
Lamotrigine
Lamotrigine, marketed in the US and most of Europe as Lamictal by GlaxoSmithKline, is an anticonvulsant drug used in the treatment of epilepsy and bipolar disorder. It is also used as an adjunct in treating depression, though this is considered off-label usage...
(Lamictal), and the inhalation anesthetic isoflurane
Isoflurane
Isoflurane is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula...
.
Rachel Carson
Rachel Carson
Rachel Louise Carson was an American marine biologist and conservationist whose writings are credited with advancing the global environmental movement....
brought the issue of DDT pesticide toxicity to public awareness with her 1962 book Silent Spring
Silent Spring
Silent Spring is a book written by Rachel Carson and published by Houghton Mifflin on 27 September 1962. The book is widely credited with helping launch the environmental movement....
. While many countries have phased out the use of some types of organochlorides such as the US ban on DDT, persistent DDT, PCBs, and other organochloride residues continue to be found in humans and mammals across the planet many years after production and use have been limited. In Arctic
Arctic
The Arctic is a region located at the northern-most part of the Earth. The Arctic consists of the Arctic Ocean and parts of Canada, Russia, Greenland, the United States, Norway, Sweden, Finland, and Iceland. The Arctic region consists of a vast, ice-covered ocean, surrounded by treeless permafrost...
areas, particularly high levels are found in marine mammal
Marine mammal
Marine mammals, which include seals, whales, dolphins, and walruses, form a diverse group of 128 species that rely on the ocean for their existence. They do not represent a distinct biological grouping, but rather are unified by their reliance on the marine environment for feeding. The level of...
s. These chemicals concentrate in mammals, and are even found in human breast milk. Males typically have far higher levels, as females reduce their concentration by transfer to their offspring through breast feeding.