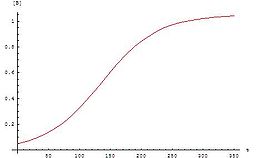
Induction period
Encyclopedia
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
is an initial slow stage of a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
; after the induction period, the reaction accelerates. Ignoring induction periods can lead to runaway reactions.
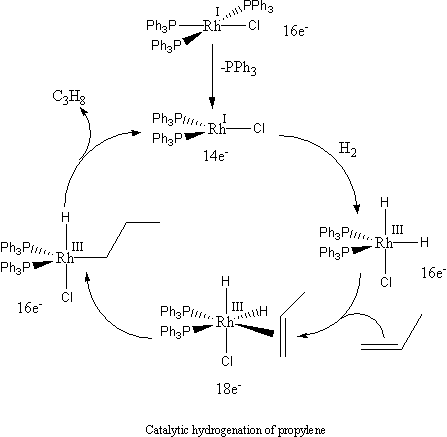
In some catalytic reactions, a pre-catalyst needs to undergo a transformation to form the active catalyst, before the catalyst can take effect. Time is required for this transformation, hence the induction period. For example, with Wilkinson's catalyst
Wilkinson's catalyst
Wilkinson's catalyst is the common name for chlorotrisrhodium, a coordination compound with the formula RhCl3 . It is named after the late organometallic chemist and 1973 Nobel Laureate, Sir Geoffrey Wilkinson who popularized its use.-Structure and basic properties:The compound is a square planar,...
, one triphenylphosphine ligand must dissociate to give the coordinatively unsaturated 14-electron species which can participate in the catalytic cycle:
Similarly, for an autocatalytic reaction
Autocatalytic reaction
Autocatalytic reactions are chemical reactions in which at least one of the reactants is also a product. The rate equations for autocatalytic reactions are fundamentally nonlinear. This nonlinearity can lead to the spontaneous generation of order. A dramatic example of this order is that which is...
, where one of the reaction products catalyzes the reaction itself, the rate of reaction is low initially until sufficient products have formed to catalyze the reaction.
Reactions generally accelerate when heat is applied. Where a reaction is exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
, the rate of the reaction may initially be low. As the reaction proceeds, heat is generated, and the rate of reaction increases. This type of reaction often exhibits an induction period as well.
Grignard reagents are notorious for having induction periods. This is usually due to two reasons: Firstly, the thin film of oxide on the magnesium reagent must be removed before the bulk magnesium can react. Secondly, Grignard reactions, while exothermic, are typically conducted at low temperature for better selectivity. For these two reasons, Grignard reactions often can have a long induction period, followed by a thermal runaway
Thermal runaway
Thermal runaway refers to a situation where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result...
, even causing the reaction solvent to boil-off.