Gamma spectroscopy
Encyclopedia
Gamma-ray spectroscopy is the quantitative study of the energy spectra of gamma-ray sources, both nuclear laboratory, geochemical, and astrophysical. Gamma rays are the highest-energy form of electromagnetic radiation
, being physically exactly like all other forms (e.g., X rays, visible light, infrared, radio) except for higher photon
energy and frequency, and shorter wavelength. (Because of their high energy, gamma-ray photons are generally counted individually, whereas the lowest energy forms of EM radiation (e.g., radio to sub-millimeter) are observed as electromagnetic waves consisting of many low-energy photons.) While a Geiger counter
or Gamma Probe
determine only the count rate (i.e. the number of gamma rays interacting in the detector in one second), a gamma-ray spectrometer also determines the energies of the gamma-rays photons emitted by the source. Radioactive nuclei (radionuclides) commonly emit gamma rays in the energy range from a few keV to ~10 MeV, corresponding to the typical energy levels in nuclei with reasonably long lifetimes. Such sources typically produce gamma-ray "line spectra" (i.e., many photon
s emitted at discrete energies), whereas much higher energies (upwards of 1 TeV
) may occur in the continuum spectra observed in astrophysics and elementary particle physics. The boundary between gamma rays and X rays is somewhat blurred, as X rays typically refer to the high energy EM
emission of atoms, which may extend to over 100 keV, whereas the lowest energy emissions of nuclei are typically termed gamma rays, even though their energies may be below 20 keV.
Most radioactive sources produce gamma rays of various energies and intensities. When these emissions are collected and analyzed with a gamma-ray spectroscopy system, a gamma-ray energy spectrum can be produced. A detailed analysis of this spectrum is typically used to determine the identity and quantity of gamma emitters present in the source. The gamma spectrum is characteristic of the gamma-emitting nuclide
s contained in the source, just as in optical spectroscopy, the optical spectrum is characteristic of the atoms and molecules contained in the sample.
The equipment used in gamma spectroscopy includes an energy-sensitive radiation detector, a pulse sorter (i.e., multichannel analyzer), and associated amplifiers and data readout
devices. The most common detectors include sodium iodide (NaI)
scintillation counter
s and high-purity germanium
detectors.
Gamma spectroscopy detectors are passive materials that wait for a gamma interaction to occur in the detector volume. The most important interaction mechanisms are the photoelectric effect
, the Compton effect, and pair production. The photoelectric effect is preferred, as it absorbs all of the energy of the incident gamma ray. Full energy absorption is also possible when a series of these interaction mechanisms take place within the detector volume. When a gamma ray undergoes a Compton interaction or pair production, and a portion of the energy escapes from the detector volume without being absorbed, the background rate in the spectrum is increased by one count. This count will appear in a channel below the channel that corresponds to the full energy of the gamma ray. Larger detector volumes reduce this effect.
The voltage pulse produced by the detector (or by the photomultiplier
in a scintillation detector) is shaped by a multichannel analyzer (MCA). The multichannel analyzer takes the very small voltage signal produced by the detector, reshapes it into a Gaussian
or trapezoidal shape, and converts that signal into a digital signal. In some systems, the analog-to-digital conversion
is performed before the peak is reshaped. The analog-to-digital converter (ADC) also sorts the pulses by their height. ADCs have specific numbers of "bins" into which the pulses can be sorted; these bins represent the channels in the spectrum. The number of channels can be changed in most modern gamma spectroscopy systems by modifying software or hardware settings. The number of channels is typically a power of two; common values include 512, 1024, 2048, 4096, 8192, or 16384 channels. The choice of number of channels depends on the resolution of the system and the energy range being studied.
The multichannel analyzer output is sent to a computer, which stores, displays, and analyzes the data. A variety of software packages are available from several manufacturers, and generally include spectrum analysis tools such as energy calibration, peak area and net area calculation, and resolution calculation.
The most common figure used to express detector resolution is full width at half maximum
(FWHM). This is the width of the gamma ray peak at half of the highest point on the peak distribution. Resolution figures are given with reference to specified gamma ray energies. Resolution can be expressed in absolute (i.e., eV or MeV) or relative terms. For example, a sodium iodide (NaI) detector may have a FWHM of 9.15 keV at 122 keV, and 82.75 keV at 662 keV. These resolution values are expressed in absolute terms. To express the resolution in relative terms, the FWHM in eV or MeV is divided by the energy of the gamma ray and multiplied by 100. Using the preceding example, the resolution of the detector is 7.5% at 122 keV, and 12.5% at 662 keV. A germanium detector may give resolution of 560 eV at 122 keV, yielding a relative resolution of 0.46%.
Efficiency, like resolution, can be expressed in absolute or relative terms. The same units are used (i.e., percentages); therefore, the spectroscopist must take care to determine which kind of efficiency is being given for the detector. Absolute efficiency values represent the probability that a gamma ray of a specified energy passing through the detector will interact and be detected. Relative efficiency values are often used for germanium detectors, and compare the efficiency of the detector at 1332 keV to that of a 3 in × 3 in NaI detector (i.e., 1.2×10−3 cps
/Bq
at 25 cm). Relative efficiency values greater than one hundred percent can therefore be encountered when working with very large germanium detectors.
The energy of the gamma rays being detected is an important factor in the efficiency of the detector. An efficiency curve can be obtained by plotting the efficiency at various energies. This curve can then be used to determine the efficiency of the detector at energies different from those used to obtain the curve. High-purity germanium (HPGe) detectors typically have higher sensitivity.
use crystals that emit light when gamma rays interact with the atoms in the crystals. The intensity of the light produced is proportional to the energy deposited in the crystal by the gamma ray. The mechanism is similar to that of a thermoluminescent dosimeter
. The detectors are joined to photomultiplier
s that convert the light into electrons and then amplify the electrical signal provided by those electrons. Common scintillators include thallium
-doped sodium iodide
(NaI(Tl))—often simplified to sodium iodide (NaI) detectors—and bismuth germanate
(BGO). Because photomultipliers are also sensitive to ambient light, scintillators are encased in light-tight coverings.
Scintillation detectors can also be used to detect alpha- and beta-radiation.
 Thallium-doped sodium iodide (NaI(Tl)) has two principal advantages:
Thallium-doped sodium iodide (NaI(Tl)) has two principal advantages:
NaI(Tl) is also convenient to use, making it popular for field applications such as the identification of unknown materials for law enforcement purposes.
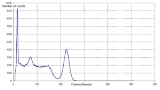
An example of a NaI spectrum is the gamma spectrum of the caesium
isotope 137Cs—see Figure 1. 137Cs emits a single gamma line of 662 keV. It should be noted that the 662 keV line shown is actually produced by 137Ba
m, the decay product
of 137Cs, which is in secular equilibrium
with 137Cs.
The spectrum in Figure 1 was measured using a NaI-crystal on a photomultiplier, an amplifier, and a multichannel analyzer. The figure shows the number of counts (within the measuring period) versus channel number. The spectrum indicates the following peaks (from left to right):
The Compton distribution is a continuous distribution that is present up to channel 150 in Figure 1. The distribution arises because of primary gamma rays undergoing Compton scattering
within the crystal: Depending on the scattering angle, the Compton electrons have different energies and hence produce pulses of different heights.
If many gamma rays are present in a spectrum, Compton distributions can present analysis challenges. To reduce gamma rays, an anticoincidence shield can be used—see Compton suppression
. Gamma ray reduction techniques are especially useful for small lithium
-doped germanium (Ge(Li)) detectors.
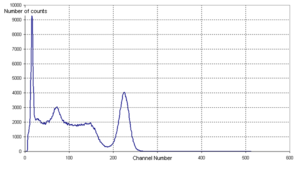
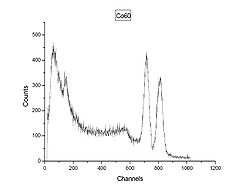
The gamma spectrum shown in Figure 2 is of the cobalt
isotope 60Co, with two gamma rays with 1.17 MeV and 1.33 MeV respectively. (See the decay scheme
article for the decay scheme of cobalt-60.) The two gamma lines can be seen well-separated; the peak to the left of channel 200 most likely indicates a strong background radiation
source that has not been subtracted. A backscatter peak can be seen at channel 150, similar to the second peak in Figure 1.
Sodium iodide systems, as with all scintillator systems, are sensitive to changes in temperature. Changes in the operating temperature
caused by changes in environmental temperature will shift the spectrum on the horizontal axis. Peak shifts of tens of channels or more are commonly observed. Such shifts can be prevented by using spectrum stabilizers.
Because of the poor resolution of NaI-based detectors, they are not suitable for the identification of complicated mixtures of gamma ray-producing materials. Scenarios requiring such analyses require detectors with higher resolution.
 Semiconductor detector
Semiconductor detector
s, also called solid-state detectors, are fundamentally different from scintillation detectors: They rely on detection of the charge carriers (electrons and holes) generated in semiconductors by energy deposited by gamma ray photons.
In semiconductor detectors, an electric field is applied to the detector volume. An electron in the semiconductor is fixed in its valence band
in the crystal until a gamma ray interaction provides the electron enough energy to move to the conduction band
. Electrons in the conduction band can respond to the electric field in the detector, and therefore move to the positive contact that is creating the electrical field. The gap created by the moving electron is called a "hole," and is filled by an adjacent electron. This shuffling of holes effectively moves a positive charge to the negative contact. The arrival of the electron at the positive contact and the hole at the negative contact produces the electrical signal that is sent to the preamplifier, the MCA, and on through the system for analysis. The movement of electrons and holes in a solid-state detector is very similar to the movement of ions within the sensitive volume of gas-filled detectors such as ionization chamber
s.
Common semiconductor-based detectors include germanium
, cadmium telluride
, and cadmium zinc telluride
.
Germanium detectors provide significantly improved energy resolution in comparison to sodium iodide detectors, as explained in the preceding discussion of resolution. Germanium detectors produce the highest resolution commonly available today. Cryogenic temperatures are vital to the operation of germanium detectors.
Because some radioactivity is present everywhere (i.e., background radiation
), the spectrum should be analyzed when no source is present. The background radiation must then be subtracted from the actual measurement. Lead
absorbers can be placed around the measurement apparatus to reduce background radiation.
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
, being physically exactly like all other forms (e.g., X rays, visible light, infrared, radio) except for higher photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
energy and frequency, and shorter wavelength. (Because of their high energy, gamma-ray photons are generally counted individually, whereas the lowest energy forms of EM radiation (e.g., radio to sub-millimeter) are observed as electromagnetic waves consisting of many low-energy photons.) While a Geiger counter
Geiger counter
A Geiger counter, also called a Geiger–Müller counter, is a type of particle detector that measures ionizing radiation. They detect the emission of nuclear radiation: alpha particles, beta particles or gamma rays. A Geiger counter detects radiation by ionization produced in a low-pressure gas in a...
or Gamma Probe
Gamma Probe
A gamma probe is a handheld device used with a Geiger-Muller tube or scintillation counter, for intraoperative use following interstitial injection of a radionuclide, to locate regional lymph nodes by their radioactivity...
determine only the count rate (i.e. the number of gamma rays interacting in the detector in one second), a gamma-ray spectrometer also determines the energies of the gamma-rays photons emitted by the source. Radioactive nuclei (radionuclides) commonly emit gamma rays in the energy range from a few keV to ~10 MeV, corresponding to the typical energy levels in nuclei with reasonably long lifetimes. Such sources typically produce gamma-ray "line spectra" (i.e., many photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s emitted at discrete energies), whereas much higher energies (upwards of 1 TeV
TEV
TEV may refer to:* TeV, or teraelectronvolt, a measure of energy* Total Enterprise Value, a financial measure* Total Economic Value, an economic measure* Tobacco etch virus, a plant pathogenic virus of the family Potyviridae....
) may occur in the continuum spectra observed in astrophysics and elementary particle physics. The boundary between gamma rays and X rays is somewhat blurred, as X rays typically refer to the high energy EM
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
emission of atoms, which may extend to over 100 keV, whereas the lowest energy emissions of nuclei are typically termed gamma rays, even though their energies may be below 20 keV.
Most radioactive sources produce gamma rays of various energies and intensities. When these emissions are collected and analyzed with a gamma-ray spectroscopy system, a gamma-ray energy spectrum can be produced. A detailed analysis of this spectrum is typically used to determine the identity and quantity of gamma emitters present in the source. The gamma spectrum is characteristic of the gamma-emitting nuclide
Nuclide
A nuclide is an atomic species characterized by the specific constitution of its nucleus, i.e., by its number of protons Z, its number of neutrons N, and its nuclear energy state....
s contained in the source, just as in optical spectroscopy, the optical spectrum is characteristic of the atoms and molecules contained in the sample.
The equipment used in gamma spectroscopy includes an energy-sensitive radiation detector, a pulse sorter (i.e., multichannel analyzer), and associated amplifiers and data readout
Data acquisition
Data acquisition is the process of sampling signals that measure real world physical conditions and converting the resulting samples into digital numeric values that can be manipulated by a computer. Data acquisition systems typically convert analog waveforms into digital values for processing...
devices. The most common detectors include sodium iodide (NaI)
Scintillator
A scintillator is a special material, which exhibits scintillation—the property of luminescence when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate, i.e., reemit the absorbed energy in the form of light...
scintillation counter
Scintillation counter
A scintillation counter measures ionizing radiation. The sensor, called a scintillator, consists of a transparent crystal, usually phosphor, plastic , or organic liquid that fluoresces when struck by ionizing radiation. A sensitive photomultiplier tube measures the light from the crystal...
s and high-purity germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
detectors.
System components
A gamma spectroscopy system consists of a detector, electronics to collect and process the signals produced by the detector, and a computer with processing software to generate, display, and store the spectrum. Other components, such as rate meters and peak position stabilizers, may also be included.Gamma spectroscopy detectors are passive materials that wait for a gamma interaction to occur in the detector volume. The most important interaction mechanisms are the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
, the Compton effect, and pair production. The photoelectric effect is preferred, as it absorbs all of the energy of the incident gamma ray. Full energy absorption is also possible when a series of these interaction mechanisms take place within the detector volume. When a gamma ray undergoes a Compton interaction or pair production, and a portion of the energy escapes from the detector volume without being absorbed, the background rate in the spectrum is increased by one count. This count will appear in a channel below the channel that corresponds to the full energy of the gamma ray. Larger detector volumes reduce this effect.
The voltage pulse produced by the detector (or by the photomultiplier
Photomultiplier
Photomultiplier tubes , members of the class of vacuum tubes, and more specifically phototubes, are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum...
in a scintillation detector) is shaped by a multichannel analyzer (MCA). The multichannel analyzer takes the very small voltage signal produced by the detector, reshapes it into a Gaussian
GAUSSIAN
Gaussian is a computational chemistry software program initially released in 1970 by John Pople and his research group at Carnegie-Mellon University as Gaussian 70. It has been continuously updated since then...
or trapezoidal shape, and converts that signal into a digital signal. In some systems, the analog-to-digital conversion
Analog-to-digital converter
An analog-to-digital converter is a device that converts a continuous quantity to a discrete time digital representation. An ADC may also provide an isolated measurement...
is performed before the peak is reshaped. The analog-to-digital converter (ADC) also sorts the pulses by their height. ADCs have specific numbers of "bins" into which the pulses can be sorted; these bins represent the channels in the spectrum. The number of channels can be changed in most modern gamma spectroscopy systems by modifying software or hardware settings. The number of channels is typically a power of two; common values include 512, 1024, 2048, 4096, 8192, or 16384 channels. The choice of number of channels depends on the resolution of the system and the energy range being studied.
The multichannel analyzer output is sent to a computer, which stores, displays, and analyzes the data. A variety of software packages are available from several manufacturers, and generally include spectrum analysis tools such as energy calibration, peak area and net area calculation, and resolution calculation.
Detector performance
Gamma spectroscopy systems are selected to take advantage of several performance characteristics. Two of the most important include detector resolution and detector efficiency.Detector resolution
Gamma rays detected in a spectroscopic system produce peaks in the spectrum. These peaks can also be called lines by analogy to optical spectroscopy. The width of the peaks is determined by the resolution of the detector, a very important characteristic of gamma spectroscopic detectors, and high resolution enables the spectroscopist to separate two gamma lines that are close to each other. Gamma spectroscopy systems are designed and adjusted to produce symmetrical peaks of the best possible resolution. The peak shape is usually a Gaussian distribution. In most spectra the horizontal position of the peak is determined by the gamma ray's energy, and the area of the peak is determined by the intensity of the gamma ray and the efficiency of the detector.The most common figure used to express detector resolution is full width at half maximum
Full width at half maximum
Full width at half maximum is an expression of the extent of a function, given by the difference between the two extreme values of the independent variable at which the dependent variable is equal to half of its maximum value....
(FWHM). This is the width of the gamma ray peak at half of the highest point on the peak distribution. Resolution figures are given with reference to specified gamma ray energies. Resolution can be expressed in absolute (i.e., eV or MeV) or relative terms. For example, a sodium iodide (NaI) detector may have a FWHM of 9.15 keV at 122 keV, and 82.75 keV at 662 keV. These resolution values are expressed in absolute terms. To express the resolution in relative terms, the FWHM in eV or MeV is divided by the energy of the gamma ray and multiplied by 100. Using the preceding example, the resolution of the detector is 7.5% at 122 keV, and 12.5% at 662 keV. A germanium detector may give resolution of 560 eV at 122 keV, yielding a relative resolution of 0.46%.
Detector efficiency
Not all gamma rays emitted by the source and pass through the detector will produce a count in the system. The probability that an emitted gamma ray will interact with the detector and produce a count is the efficiency of the detector. High-efficiency detectors produce spectra in less time than low-efficiency detectors. In general, larger detectors have higher efficiency than smaller detectors, although the shielding properties of the detector material are also important factors. Detector efficiency is measured by comparing a spectrum from a source of known activity to the count rates in each peak to the count rates expected from the known intensities of each gamma ray.Efficiency, like resolution, can be expressed in absolute or relative terms. The same units are used (i.e., percentages); therefore, the spectroscopist must take care to determine which kind of efficiency is being given for the detector. Absolute efficiency values represent the probability that a gamma ray of a specified energy passing through the detector will interact and be detected. Relative efficiency values are often used for germanium detectors, and compare the efficiency of the detector at 1332 keV to that of a 3 in × 3 in NaI detector (i.e., 1.2×10−3 cps
Second
The second is a unit of measurement of time, and is the International System of Units base unit of time. It may be measured using a clock....
/Bq
Becquerel
The becquerel is the SI-derived unit of radioactivity. One Bq is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. The Bq unit is therefore equivalent to an inverse second, s−1...
at 25 cm). Relative efficiency values greater than one hundred percent can therefore be encountered when working with very large germanium detectors.
The energy of the gamma rays being detected is an important factor in the efficiency of the detector. An efficiency curve can be obtained by plotting the efficiency at various energies. This curve can then be used to determine the efficiency of the detector at energies different from those used to obtain the curve. High-purity germanium (HPGe) detectors typically have higher sensitivity.
Scintillation detectors
Scintillation detectorsScintillator
A scintillator is a special material, which exhibits scintillation—the property of luminescence when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate, i.e., reemit the absorbed energy in the form of light...
use crystals that emit light when gamma rays interact with the atoms in the crystals. The intensity of the light produced is proportional to the energy deposited in the crystal by the gamma ray. The mechanism is similar to that of a thermoluminescent dosimeter
Thermoluminescent Dosimeter
A thermoluminescent dosimeter, or TLD, is a type of radiation dosimeter. A TLD measures ionizing radiation exposure by measuring the amount of visible light emitted from a crystal in the detector when the crystal is heated. The amount of light emitted is dependent upon the radiation exposure...
. The detectors are joined to photomultiplier
Photomultiplier
Photomultiplier tubes , members of the class of vacuum tubes, and more specifically phototubes, are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum...
s that convert the light into electrons and then amplify the electrical signal provided by those electrons. Common scintillators include thallium
Thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
-doped sodium iodide
Sodium iodide
Sodium iodide is a white, crystalline salt with chemical formula NaI used in radiation detection, treatment of iodine deficiency, and as a reactant in the Finkelstein reaction.-Uses:Sodium iodide is commonly used to treat and prevent iodine deficiency....
(NaI(Tl))—often simplified to sodium iodide (NaI) detectors—and bismuth germanate
Bismuth germanate
Bismuth germanium oxide is an inorganic chemical compound with main use as a scintillator. It forms cubic crystals....
(BGO). Because photomultipliers are also sensitive to ambient light, scintillators are encased in light-tight coverings.
Scintillation detectors can also be used to detect alpha- and beta-radiation.
Sodium iodide-based detectors


- It can be produced in large crystals, yielding good efficiency, and
- it produces intense bursts of light compared to other spectroscopic scintillators.
NaI(Tl) is also convenient to use, making it popular for field applications such as the identification of unknown materials for law enforcement purposes.
An example of a NaI spectrum is the gamma spectrum of the caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
isotope 137Cs—see Figure 1. 137Cs emits a single gamma line of 662 keV. It should be noted that the 662 keV line shown is actually produced by 137Ba
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
m, the decay product
Decay product
In nuclear physics, a decay product is the remaining nuclide left over from radioactive decay. Radioactive decay often involves a sequence of steps...
of 137Cs, which is in secular equilibrium
Secular equilibrium
In nuclear physics, secular equilibrium is a situation in which the quantity of a radioactive isotope remains constant because its production rate is equal to its decay rate.-Secular equilibrium in radioactive decay:...
with 137Cs.
The spectrum in Figure 1 was measured using a NaI-crystal on a photomultiplier, an amplifier, and a multichannel analyzer. The figure shows the number of counts (within the measuring period) versus channel number. The spectrum indicates the following peaks (from left to right):
- low energy x radiation (due to internal conversionInternal conversionInternal conversion is a radioactive decay process where an excited nucleus interacts with an electron in one of the lower atomic orbitals, causing the electron to be emitted from the atom. Thus, in an internal conversion process, a high-energy electron is emitted from the radioactive atom, but...
of the gamma ray), - backscatterBackscatterIn physics, backscatter is the reflection of waves, particles, or signals back to the direction they came from. It is a diffuse reflection due to scattering, as opposed to specular reflection like a mirror...
at the low energy end of the Compton distribution, and - a photopeak (full energy peak) at an energy of 662 keV
The Compton distribution is a continuous distribution that is present up to channel 150 in Figure 1. The distribution arises because of primary gamma rays undergoing Compton scattering
Compton scattering
In physics, Compton scattering is a type of scattering that X-rays and gamma rays undergo in matter. The inelastic scattering of photons in matter results in a decrease in energy of an X-ray or gamma ray photon, called the Compton effect...
within the crystal: Depending on the scattering angle, the Compton electrons have different energies and hence produce pulses of different heights.
If many gamma rays are present in a spectrum, Compton distributions can present analysis challenges. To reduce gamma rays, an anticoincidence shield can be used—see Compton suppression
Compton suppression
Electronic anticoincidence is a method widely used to suppress unwanted, "background" events in high energy physics, experimental particle physics, gamma-ray spectroscopy, gamma-ray astronomy, experimental nuclear physics, and related fields...
. Gamma ray reduction techniques are especially useful for small lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
-doped germanium (Ge(Li)) detectors.
The gamma spectrum shown in Figure 2 is of the cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
isotope 60Co, with two gamma rays with 1.17 MeV and 1.33 MeV respectively. (See the decay scheme
Decay scheme
The Decay scheme of a radioactive substance is a graphical presentation of all the transitions occurring in a decay, and of their relationships.-Decay schemes of radioactive isotopes:...
article for the decay scheme of cobalt-60.) The two gamma lines can be seen well-separated; the peak to the left of channel 200 most likely indicates a strong background radiation
Background radiation
Background radiation is the ionizing radiation constantly present in the natural environment of the Earth, which is emitted by natural and artificial sources.-Overview:Both Natural and human-made background radiation varies by location....
source that has not been subtracted. A backscatter peak can be seen at channel 150, similar to the second peak in Figure 1.
Sodium iodide systems, as with all scintillator systems, are sensitive to changes in temperature. Changes in the operating temperature
Operating temperature
An operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
caused by changes in environmental temperature will shift the spectrum on the horizontal axis. Peak shifts of tens of channels or more are commonly observed. Such shifts can be prevented by using spectrum stabilizers.
Because of the poor resolution of NaI-based detectors, they are not suitable for the identification of complicated mixtures of gamma ray-producing materials. Scenarios requiring such analyses require detectors with higher resolution.
Semiconductor-based detectors

Semiconductor detector
This article is about particle detectors. For information about semiconductor detectors in radio, see Diode#Semiconductor_diodes, rectifier, detector and cat's-whisker detector....
s, also called solid-state detectors, are fundamentally different from scintillation detectors: They rely on detection of the charge carriers (electrons and holes) generated in semiconductors by energy deposited by gamma ray photons.
In semiconductor detectors, an electric field is applied to the detector volume. An electron in the semiconductor is fixed in its valence band
Valence band
In solids, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature....
in the crystal until a gamma ray interaction provides the electron enough energy to move to the conduction band
Conduction band
In the solid-state physics field of semiconductors and insulators, the conduction band is the range of electron energies, higher than that of the valence band, sufficient to free an electron from binding with its individual atom and allow it to move freely within the atomic lattice of the material...
. Electrons in the conduction band can respond to the electric field in the detector, and therefore move to the positive contact that is creating the electrical field. The gap created by the moving electron is called a "hole," and is filled by an adjacent electron. This shuffling of holes effectively moves a positive charge to the negative contact. The arrival of the electron at the positive contact and the hole at the negative contact produces the electrical signal that is sent to the preamplifier, the MCA, and on through the system for analysis. The movement of electrons and holes in a solid-state detector is very similar to the movement of ions within the sensitive volume of gas-filled detectors such as ionization chamber
Ionization chamber
The ionization chamber is the simplest of all gas-filled radiation detectors, and is used for the detection or measurement of ionizing radiation...
s.
Common semiconductor-based detectors include germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
, cadmium telluride
Cadmium telluride
Cadmium telluride is a crystalline compound formed from cadmium and tellurium. It is used as an infrared optical window and a solar cell material. It is usually sandwiched with cadmium sulfide to form a p-n junction photovoltaic solar cell...
, and cadmium zinc telluride
Cadmium zinc telluride
Cadmium zinc telluride, or CZT, is a compound of cadmium, zinc and tellurium or, more strictly speaking, an alloy of cadmium telluride and zinc telluride. A direct bandgap semiconductor, it is used in a variety of applications, including radiation detectors, photorefractive gratings,...
.
Germanium detectors provide significantly improved energy resolution in comparison to sodium iodide detectors, as explained in the preceding discussion of resolution. Germanium detectors produce the highest resolution commonly available today. Cryogenic temperatures are vital to the operation of germanium detectors.
Calibration and background radiation
If a gamma spectrometer is used for identifying samples of unknown composition, its energy scale must be calibrated first. Calibration is performed by using the peaks of a known source, such as cesium-137 or cobalt-60. Because the channel number is proportional to energy, the channel scale can then be converted to an energy scale. If the size of the detector crystal is known, one can also perform an intensity calibration, so that not only the energies but also the intensities of an unknown source—or the amount of a certain isotope in the source—can be determined.Because some radioactivity is present everywhere (i.e., background radiation
Background radiation
Background radiation is the ionizing radiation constantly present in the natural environment of the Earth, which is emitted by natural and artificial sources.-Overview:Both Natural and human-made background radiation varies by location....
), the spectrum should be analyzed when no source is present. The background radiation must then be subtracted from the actual measurement. Lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
absorbers can be placed around the measurement apparatus to reduce background radiation.
See also
- Gamma ray spectrometerGamma ray spectrometerA Gamma-Ray Spectrometer, or , is an instrument for measuring the distribution of the intensity of gamma radiation versus the energy of each photon....
- Alpha-particle spectroscopyAlpha-particle spectroscopyOne method for testing for many alpha emitters is to use alpha-particle spectroscopy. For methods for gamma rays and beta particles, please see gamma spectroscopy and liquid scintillation counting respectively....
- Liquid scintillation countingLiquid scintillation countingLiquid scintillation counting is a standard laboratory method in the life-sciences for measuring radiation from beta-emitting nuclides. Scintillating materials are also used in differently constructed "counters" in many other fields....
- Gamma ProbeGamma ProbeA gamma probe is a handheld device used with a Geiger-Muller tube or scintillation counter, for intraoperative use following interstitial injection of a radionuclide, to locate regional lymph nodes by their radioactivity...
- Mass spectrometryMass spectrometryMass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
- X-ray spectroscopyX-ray spectroscopyX-ray spectroscopy is a gathering name for several spectroscopic techniques for characterization of materials by using x-ray excitation.-Characteristic X-ray Spectroscopy:...
- Isomeric shiftIsomeric shiftThe isomeric shift is the shift on atomic spectral lines and gamma spectral lines, which occurs as a consequence of replacement of one nuclear isomer by another. It is usually called isomeric shift on atomic spectral lines and Mössbauer isomeric shift respectively...

