
Internal conversion
Encyclopedia
Internal conversion is a radioactive decay
process where an excited nucleus
interacts with an electron
in one of the lower atomic orbital
s, causing the electron to be emitted from the atom. Thus, in an internal conversion process, a high-energy electron is emitted from the radioactive atom, but without beta decay
taking place. For this reason, the high-speed electrons from internal conversion are not beta particles (β particles), since the latter come from beta decay.
Since no beta decay takes place in internal conversion, the element atomic number does not change, and thus (as is the case with gamma decay) no transmutation of one element to another is seen. Also, no neutrino is emitted in internal conversion.
Internally converted electrons do not have the characteristic energetically-spread spectrum of β particles, which results from varying amounts of decay-energy being carried off by the neutrino (or antineutrino) in beta decay. Internally converted electrons, which carry a fixed fraction of the characteristic decay energy, have a well-specified discrete energy. The energy spectrum of a β particle is thus a broad hump, extending to a maximum decay energy value, while the spectrum of internally converted electrons is a sharp peak.
of an inner shell electron penetrates the nucleus (i.e. there is a finite probability of the electron in an s atomic orbital
being found in the nucleus) and when this is the case, the electron may couple to the excited state and take the energy of the nuclear transition directly, without an intermediate gamma ray being produced first.
As an electromagnetic quantum process, the process of imparting energy to the electron may be seen as taking place by means of a virtual photon, but in that sense the photon involved can be considered as a "virtual gamma ray", which never appears except as a feature of an equation, rather than a directly measurable particle. The kinetic energy of the emitted electron is equal to the transition energy in the nucleus, minus the binding energy of the electron.
Most internal conversion electrons come from the K shell (1s state, see electron shell
), as these two electrons have the highest probability of being found inside the nucleus. After the electron has been emitted, the atom is left with a vacancy in one of the inner electron shells. This hole will be filled with an electron from one of the higher shells and subsequently a characteristic x-ray
or Auger electron
will be emitted.
states are the same, but the multi-polarity rules for nonzero initial and final spin states do not necessarily forbid the emission of a gamma ray in such a case.
The tendency towards internal conversion can be determined by the internal conversion coefficient
, which is empirically determined by the ratio of de-excitations that go by the emission of electrons to those that go by gamma emission.
The internal conversion process competes with gamma decay. This competition is quantified in the form of the internal conversion coefficient which is defined as
 where
where  is the rate of conversion electrons and
is the rate of conversion electrons and  is the rate of gamma-ray emission observed from a decaying nucleus. For example, in the decay of an excited state of the nucleus of 125I
is the rate of gamma-ray emission observed from a decaying nucleus. For example, in the decay of an excited state of the nucleus of 125I
, 7% of the decays emit energy as a gamma ray, while 93% release energy as conversion electrons. Therefore, this excited state of has an internal conversion coefficient of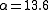 . Internal conversion coefficients are observed to increase for increasing atomic number
. Internal conversion coefficients are observed to increase for increasing atomic number
(Z) and decreasing gamma-ray energy. As one example, IC coefficients are calculated explicitly for , , 99mTc
, , 113mIn, 115mIn, , , 193mPt
, and by Howell (1992) using Monte Carlo method
s (for the IC coefficient is zero).
The energy of the emitted gamma-ray is regarded as a precise measure of the difference in energy between the excited states of the decaying nucleus. However, this is not true in the case of conversion electrons. The energy of a conversion electron is given as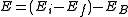 where
where  and
and 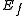 are the energies of the nucleus in its initial and final states, respectively, while
are the energies of the nucleus in its initial and final states, respectively, while 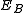 is the binding energy of the electron.
is the binding energy of the electron.
, which also may occur with gamma radiation associated electron emission, in which an incident gamma photon emitted from a nucleus interacts with an electron, expelling the electron from the atom. Thus, gamma photoelectric effect
electron emission may also cause high-speed electrons to be emitted from radioactive atoms without beta decay
. However, in internal conversion, the nucleus does not first emit an intermediate real gamma ray
, and therefore need not change angular momentum
or electric moment.
Also, electrons from the gamma photoelectric effect show a spread in energy, depending on how much energy has been imparted to the ejected electron by the gamma ray which interacts with it—an amount which is variable depending on the angle of gamma photon scattering from the electron (see Compton scattering
). Further, a gamma ray is still emitted in photoelectric processes, but one which possesses a fraction of the energy than the gamma ray which left the nucleus. By contrast, in internal conversion, as noted, no gamma ray is emitted at all and the electron energy is fixed at a single, typical value.
Auger electron
s, which may also be produced after an internal conversion, arise from a mechanism that is different from that of internal conversion, but is analogous to it. Internal conversion electrons arise when an intense electric dipole field inside the nucleus accelerates an electron which has penetrated the nucleus, to remove it from the atom. Auger electrons similarly arise when an electric field is produced within an atom's electron cloud due to loss of another electron, and this field again induces the acceleration and removal of yet another of the atom's atomic orbital
electrons. Like internal conversion electrons, Auger electrons also emerge in a sharp energy peak.
The electron capture
(EC) process also involves an inner shell electron, which in this case is retained in the nucleus (changing the atomic number) and leaving the atom (not the nucleus) in an excited state. The atom can relax by X-ray
emission and/or by Auger electron emission. Unstable nuclei can usually decay through both IC and EC processes.
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
process where an excited nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
interacts with an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
in one of the lower atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
s, causing the electron to be emitted from the atom. Thus, in an internal conversion process, a high-energy electron is emitted from the radioactive atom, but without beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
taking place. For this reason, the high-speed electrons from internal conversion are not beta particles (β particles), since the latter come from beta decay.
Since no beta decay takes place in internal conversion, the element atomic number does not change, and thus (as is the case with gamma decay) no transmutation of one element to another is seen. Also, no neutrino is emitted in internal conversion.
Internally converted electrons do not have the characteristic energetically-spread spectrum of β particles, which results from varying amounts of decay-energy being carried off by the neutrino (or antineutrino) in beta decay. Internally converted electrons, which carry a fixed fraction of the characteristic decay energy, have a well-specified discrete energy. The energy spectrum of a β particle is thus a broad hump, extending to a maximum decay energy value, while the spectrum of internally converted electrons is a sharp peak.
Mechanism
In the internal conversion process, the wavefunctionWavefunction
Not to be confused with the related concept of the Wave equationA wave function or wavefunction is a probability amplitude in quantum mechanics describing the quantum state of a particle and how it behaves. Typically, its values are complex numbers and, for a single particle, it is a function of...
of an inner shell electron penetrates the nucleus (i.e. there is a finite probability of the electron in an s atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
being found in the nucleus) and when this is the case, the electron may couple to the excited state and take the energy of the nuclear transition directly, without an intermediate gamma ray being produced first.
As an electromagnetic quantum process, the process of imparting energy to the electron may be seen as taking place by means of a virtual photon, but in that sense the photon involved can be considered as a "virtual gamma ray", which never appears except as a feature of an equation, rather than a directly measurable particle. The kinetic energy of the emitted electron is equal to the transition energy in the nucleus, minus the binding energy of the electron.
Most internal conversion electrons come from the K shell (1s state, see electron shell
Electron shell
An electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" , followed by the "2 shell" , then the "3 shell" , and so on further and further from the nucleus. The shell letters K,L,M,.....
), as these two electrons have the highest probability of being found inside the nucleus. After the electron has been emitted, the atom is left with a vacancy in one of the inner electron shells. This hole will be filled with an electron from one of the higher shells and subsequently a characteristic x-ray
Characteristic x-ray
A high energy electron interacts with a bound electron in an atom and ejects it. The incident electron is scattered and the target electron gets displaced from its shell. The incident electron energy must exceed the binding energy of the electron to eject it...
or Auger electron
Auger electron
The Auger effect is a physical phenomenon in which the transition of an electron in an atom filling in an inner-shell vacancy causes the emission of another electron. When a core electron is removed, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in...
will be emitted.
When the process is expected
Internal conversion is favoured when the energy gap between nuclear levels is small, and is also the primary mode of de-excitation for 0+→0+ (i.e. E0) transitions (i.e., where excited nuclei are able to rid themselves of energy without changing electric and magnetic moments in certain ways) with insufficient energy to decay by pair production. It is the predominant mode of de-excitation whenever the initial and final spinSpin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
states are the same, but the multi-polarity rules for nonzero initial and final spin states do not necessarily forbid the emission of a gamma ray in such a case.
The tendency towards internal conversion can be determined by the internal conversion coefficient
Internal conversion coefficient
In nuclear physics, the internal conversion coefficient describes the rate of internal conversion.The internal conversion coefficient may be empirically determined by the following formula:...
, which is empirically determined by the ratio of de-excitations that go by the emission of electrons to those that go by gamma emission.
The internal conversion process competes with gamma decay. This competition is quantified in the form of the internal conversion coefficient which is defined as
 where
where  is the rate of conversion electrons and
is the rate of conversion electrons and  is the rate of gamma-ray emission observed from a decaying nucleus. For example, in the decay of an excited state of the nucleus of 125I
is the rate of gamma-ray emission observed from a decaying nucleus. For example, in the decay of an excited state of the nucleus of 125IIodine-125
Iodine-125 is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat prostate cancer and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.Its half-life is around 59 days and it...
, 7% of the decays emit energy as a gamma ray, while 93% release energy as conversion electrons. Therefore, this excited state of has an internal conversion coefficient of
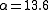 . Internal conversion coefficients are observed to increase for increasing atomic number
. Internal conversion coefficients are observed to increase for increasing atomic numberAtomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
(Z) and decreasing gamma-ray energy. As one example, IC coefficients are calculated explicitly for , , 99mTc
Technetium-99m
Technetium-99m is a metastable nuclear isomer of technetium-99, symbolized as 99mTc. The "m" indicates that this is a metastable nuclear isomer, i.e., that its half-life of 6 hours is considerably longer than most nuclear isomers that undergo gamma decay...
, , 113mIn, 115mIn, , , 193mPt
Isotopes of platinum
Natural Platinum occurs in five stable isotopes and one very-long lived radioisotope . There are also 31 known artificial radioisotopes, the longest-lived of which is 193Pt with a half-life of 50 years...
, and by Howell (1992) using Monte Carlo method
Monte Carlo method
Monte Carlo methods are a class of computational algorithms that rely on repeated random sampling to compute their results. Monte Carlo methods are often used in computer simulations of physical and mathematical systems...
s (for the IC coefficient is zero).
The energy of the emitted gamma-ray is regarded as a precise measure of the difference in energy between the excited states of the decaying nucleus. However, this is not true in the case of conversion electrons. The energy of a conversion electron is given as
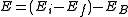 where
where  and
and 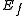 are the energies of the nucleus in its initial and final states, respectively, while
are the energies of the nucleus in its initial and final states, respectively, while 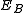 is the binding energy of the electron.
is the binding energy of the electron.Similar processes
This internal conversion process is also not to be confused with the similar photoelectric effectPhotoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
, which also may occur with gamma radiation associated electron emission, in which an incident gamma photon emitted from a nucleus interacts with an electron, expelling the electron from the atom. Thus, gamma photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
electron emission may also cause high-speed electrons to be emitted from radioactive atoms without beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
. However, in internal conversion, the nucleus does not first emit an intermediate real gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
, and therefore need not change angular momentum
Angular momentum
In physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
or electric moment.
Also, electrons from the gamma photoelectric effect show a spread in energy, depending on how much energy has been imparted to the ejected electron by the gamma ray which interacts with it—an amount which is variable depending on the angle of gamma photon scattering from the electron (see Compton scattering
Compton scattering
In physics, Compton scattering is a type of scattering that X-rays and gamma rays undergo in matter. The inelastic scattering of photons in matter results in a decrease in energy of an X-ray or gamma ray photon, called the Compton effect...
). Further, a gamma ray is still emitted in photoelectric processes, but one which possesses a fraction of the energy than the gamma ray which left the nucleus. By contrast, in internal conversion, as noted, no gamma ray is emitted at all and the electron energy is fixed at a single, typical value.
Auger electron
Auger electron
The Auger effect is a physical phenomenon in which the transition of an electron in an atom filling in an inner-shell vacancy causes the emission of another electron. When a core electron is removed, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in...
s, which may also be produced after an internal conversion, arise from a mechanism that is different from that of internal conversion, but is analogous to it. Internal conversion electrons arise when an intense electric dipole field inside the nucleus accelerates an electron which has penetrated the nucleus, to remove it from the atom. Auger electrons similarly arise when an electric field is produced within an atom's electron cloud due to loss of another electron, and this field again induces the acceleration and removal of yet another of the atom's atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
electrons. Like internal conversion electrons, Auger electrons also emerge in a sharp energy peak.
The electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
(EC) process also involves an inner shell electron, which in this case is retained in the nucleus (changing the atomic number) and leaving the atom (not the nucleus) in an excited state. The atom can relax by X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
emission and/or by Auger electron emission. Unstable nuclei can usually decay through both IC and EC processes.

