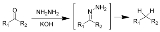
Wolff-Kishner reduction
Encyclopedia
The Wolff–Kishner reduction is a chemical reaction
that fully reduces
a ketone
(or aldehyde
) to an alkane
.
The method originally involved heating the hydrazine
with sodium ethoxide
in a sealed vessel at about 180 °C. Other bases have been found equally effective. Diethylene glycol
(DEG) is usually used as solvent.
Several reviews have been published.
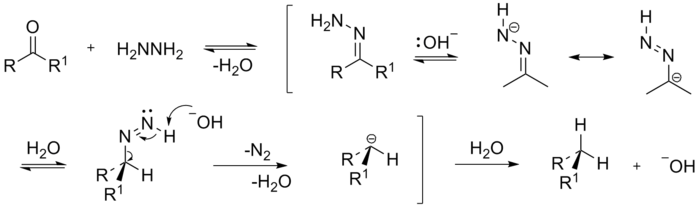 The mechanism first involves the formation of the hydrazone in a mechanism that is probably analogous to the formation of an imine
The mechanism first involves the formation of the hydrazone in a mechanism that is probably analogous to the formation of an imine
. Successive deprotonations eventually result in the evolution of nitrogen. The mechanism can be justified by the evolution of nitrogen as the thermodynamic driving force.
This reaction is also used to distinguish between aldehydes and ketones.
compound, potassium hydroxide
, and hydrazine
hydrate together in ethylene glycol
in a one-pot reaction
.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that fully reduces
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
(or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
) to an alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
.
The method originally involved heating the hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
with sodium ethoxide
Sodium ethoxide
Sodium ethoxide is an alkoxide salt with the chemical formula C2H5ONa.-Preparation:It is commercially available as a white solid, or as a solution in ethanol. It is easily prepared in the laboratory by reacting sodium metal with ethanol:...
in a sealed vessel at about 180 °C. Other bases have been found equally effective. Diethylene glycol
Diethylene glycol
Diethylene glycol is an organic compound with the formula 2O. It is a colorless, practically odorless, poisonous, and hygroscopic liquid with a sweetish taste. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent...
(DEG) is usually used as solvent.
Several reviews have been published.
Reaction mechanism
The most probable mechanism involves the elimination of an alkyl anion as the final step:
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
. Successive deprotonations eventually result in the evolution of nitrogen. The mechanism can be justified by the evolution of nitrogen as the thermodynamic driving force.
This reaction is also used to distinguish between aldehydes and ketones.
Huang-Minglon modification
The Huang-Minglon modification (after Huang Minglon) is a convenient modification of the Wolff–Kishner reduction and involves heating the carbonylCarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compound, potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
, and hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
hydrate together in ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
in a one-pot reaction
One-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor...
.
See also
- Clemmensen reductionClemmensen reductionClemmensen reduction is a chemical reaction described as a reduction of ketones to alkanes using zinc amalgam and hydrochloric acid. This reaction is named after Erik Christian Clemmensen, a Danish chemist....
- Haworth phenanthrene synthesis
- Raney nickelRaney nickelRaney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
- Wharton reactionWharton reactionThe Wharton reaction is the chemical reaction of α,β-epoxy-ketones with hydrazine to give allylic alcohols. It can be used to synthesize carenol compounds.Dupuy has developed an improved procedure.-Reaction mechanism:...
- Shapiro reactionShapiro reactionThe Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...

