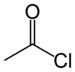
Acyl chloride
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, an acyl chloride (or acid chloride) is an organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
-CO
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
-Cl
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
. Their formula is usually written RCOCl, where R is a side chain
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
. They are usually considered to be reactive derivatives of carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s. A specific example of an acyl chloride is acetyl chloride
Acetyl chloride
Acetyl chloride, CH3COCl, also known as ethanoyl chloride or acyl chloride, is an acid chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless liquid. Acetyl chloride does not exist in nature, because contact with water would hydrolyze...
, CH3COCl. Acyl chlorides are the most important subset of acyl halide
Acyl halide
An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
s, e.g. acetyl bromide
Acetyl bromide
Acetyl bromide is an acyl bromide compound. As is expected, it may be prepared by reaction between phosphorus tribromide and acetic acid:...
.
Nomenclature
Where the acyl chloride moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting -ic acid for -yl chloride. Thus:- acetyl chlorideAcetyl chlorideAcetyl chloride, CH3COCl, also known as ethanoyl chloride or acyl chloride, is an acid chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless liquid. Acetyl chloride does not exist in nature, because contact with water would hydrolyze...
CH3COCl - benzoyl chlorideBenzoyl chlorideBenzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C6H5COCl. It is a colourless, fuming liquid with an irritating odour...
C6H5COCl
When other functional groups take priority, acyl chlorides are considered prefixes — chlorocarbonyl-:acetic acid ClOCCH2COOH
Properties
Lacking the ability to form hydrogen bondHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s, acid chlorides have lower boiling and melting points than similar carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most carbonyl compounds, infrared spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
reveals a band near 1750 cm−1.
Industrial routes
The industrial route to acetyl chloride involves the reaction of acetic anhydrideAcetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
with hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
. For benzoyl chloride, the partial hydrolysis of benzotrichloride
Benzotrichloride
Benzotrichloride, also known as trichlorotoluene is an organic compound with the formula C6H5CCl3. It is principally used as an intermediate in the preparation of other chemical products such as dyes.-Production and uses:...
is useful:
- C6H5CCl3 + H2O → C6H5C(O)Cl + 2HCl
Laboratory methods
In the laboratory, acyl chlorides are generally prepared in the same manner as alkyl chlorides, by replacing the corresponding hydroxy substituents with chlorides. Thus, carboxylic acids are treated with thionyl chlorideThionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
(SOCl2), phosphorus trichloride
Phosphorus trichloride
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having chemical formula PCl3. Its shape is trigonal pyramidal. It is the most important of the three phosphorus chlorides. It is an important industrial chemical, being used for the manufacture of organophosphorus compounds...
(PCl3), or phosphorus pentachloride (PCl5):
- RCOOH + SOCl2 → RCOCl + SO2 + HCl
- 3 RCOOH + PCl3 → 3 RCOCl + H3PO3
- RCOOH + PCl5 → RCOCl + POCl3 + HCl
The reaction with thionyl chloride may be catalyzed by dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
. In this reaction, the sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
(SO2) and hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
(HCl) generated are both gases that can leave the reaction vessel, driving the reaction forward. Excess thionyl chloride (b.p. 79 °C) is easily evaporated as well. The reaction mechanisms involving thionyl chloride and phosphorus pentachloride are similar; the mechanism with thionyl chloride is illustrative:
Another method involves the use of oxalyl chloride
Oxalyl chloride
Oxalyl chloride or ethanedioyl dichloride is a chemical compound with the formula 2. This colourless, sharp-smelling liquid, the diacid chloride of oxalic acid, is a useful reagent in organic synthesis...
:
- RCOOH + ClCOCOCl → RCOCl + CO + CO2 + HCl
The reaction is catalysed by dimethylformamide (DMF), which reacts with oxalyl chloride in the first step to give the iminium intermediate.
The iminium intermediate reacts with the carboxylic acid, abstracting an oxide, and regenerating the DMF catalyst.
Finally, methods that do not form HCl are also known, such as the Appel reaction
Appel reaction
The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using methyl iodide or iodine gives alkyl iodides...
:
- RCOOH + Ph3P + CCl4 → RCOCl + Ph3PO + HCCl3
and the use of cyanuric chloride
Cyanuric chloride
Cyanuric chloride is the inorganic compound with the formula 3. This colorless solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride...
(C3N3Cl3):
Nucleophilic reactions
Acyl chlorides are very reactive. Consider the comparison to its RCOOH acid analogue: the chloride ion is an excellent leaving group while the hydroxide is not under normal conditions; i.e. even weak nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s attack the carbonyl. A common reaction which is usually a nuisance is in fact with water yielding the carboxylic acid:
- ROCl + H2O → RO2H + HCl
Acyl chlorides can be used to prepare carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
derivatives, including acid anhydrides, ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s, and amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s by reacting acid chlorides with: a salt of a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
, an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
, or an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
respectively. The use of a base, e.g. aqueous sodium hydroxide or pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, or excess amine (when preparing amides) is desirable to remove the hydrogen chloride byproduct, and to catalyze the reaction. While it is often possible to obtain esters or amides from the carboxylic acid with alcohols or amines, the reactions are reversible, often leading to low yields. In contrast, both reactions involved in preparing esters and amides via acyl chlorides (acyl chloride formation from carboxylic acid, followed by coupling with the alcohol or amine) are fast and irreversible. This makes the two-step route often preferable to the single step reaction with the carboxylic acid.
With carbon nucleophiles such as Grignard reagents, acyl chlorides generally react first to give the ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
and then with a second equivalent to the tertiary alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. A notable exception is the reaction of acyl halides with certain organocadmium reagents which stops at the ketone stage. The nucleophilic reaction with Gilman reagent
Gilman reagent
A Gilman reagent is a lithium and copper reagent compound, R2CuLi, where R is an organic radical. These are useful because they react with organic chlorides, bromides, and iodides to replace the halide group with an R group. This is extremely useful in creating larger molecules from smaller...
s (lithium diorganocopper compounds) also afford ketones, due to their lesser reactivity. Acid chlorides of aromatic acids are generally less reactive those of alkyl acids and thus somewhat more rigorous conditions are required for reaction.
Acyl chlorides are reduced by strong hydride donors such as lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
and diisobutylaluminium hydride
Diisobutylaluminium hydride
Diisobutylaluminium hydride, DIBAL, DIBAL-H or DIBAH, is a reducing agent with the formula 2, where i-Bu represents isobutyl...
to give primary alcohols. Lithium tri-tert-butoxyaluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
, a bulky hydride donor, reduces acyl chlorides to aldehydes, as does the Rosenmund reduction
Rosenmund reduction
The Rosenmund reduction is a chemical reaction that reduces an acid halide to an aldehyde using hydrogen gas over palladium-on-carbon poisoned with barium sulfate...
using hydrogen gas over a poisoned palladium catalyst.
Electrophilic reactions
With Lewis acidLewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
catalysts like ferric chloride or aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
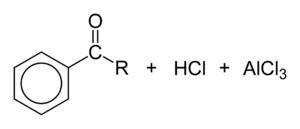
, acyl chlorides participate in Friedel-Crafts acylations, to give aryl ketones:
The first step is the Lewis acid-induced dissociation of the chloride:
This step is followed by nucleophilic attack of the arene toward the acyl group:
Finally, a chloride atom combines with the released proton to form HCl, and the AlCl3 catalyst is regenerated:
Because of the harsh conditions and the reactivity of the intermediates, this otherwise quite useful reaction tends to be messy, as well as toxic to the health and environment.