Caesium chloride
Encyclopedia
Caesium chloride is the inorganic compound
with the formula Cs
Cl
. This colorless solid is an important source of caesium
ion
s in a variety of applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chlorine ions. Caesium chloride crystals are thermally stable, but easily dissolve in water and concentrated hydrochloric acid
, and therefore gradually disintegrate in the ambient conditions due to moisture. Caesium chloride occurs naturally in mineral waters and as an impurity in carnallite
(up to 0.002%), sylvite
and kainite
. Less than 20 tonne
s of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite
.
Caesium chloride is widely used in isopycnic centrifugation
for separating various types of DNA
. It is a reagent in analytical chemistry
, where it is used to identify ions by the color and morphology of the precipitate. When enriched in radioisotopes, such as 137CsCl or 131CsCl, caesium chloride is used in nuclear medicine
applications such as treatment of cancer
and diagnosis of myocardial infarction
. Another form of cancer treatment was studied using conventional non-radioactive CsCl. Whereas conventional caesium chloride has a rather low toxicity to humans and animals, the radioactive form easily contaminates the environment due to the high solubility of CsCl in water. Spread of 137CsCl powder from a 93-gram container in 1987 in Goiânia
, Brazil, resulted in one of the worst-ever radiation spill accidents killing four and directly affecting more than 100,000 people.
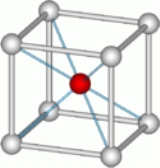
 The caesium chloride structure adopts a primitive cubic lattice with a two-atom basis, where both atoms have eightfold coordination. The chloride atoms lie upon the lattice points at the edges of the cube, while the caesium atoms lie in the holes in the center of the cubes. This structure is shared with CsBr
The caesium chloride structure adopts a primitive cubic lattice with a two-atom basis, where both atoms have eightfold coordination. The chloride atoms lie upon the lattice points at the edges of the cube, while the caesium atoms lie in the holes in the center of the cubes. This structure is shared with CsBr
and CsI
and many binary metallic alloy
s. In contrast, the other alkaline halides have the sodium chloride
(rocksalt) structure. When both ions are similar in size (Cs+ ionic radius
174 pm for this coordination number, Cl− 181 pm) the CsCl structure is adopted, when they are different (Na+ ionic radius
102 pm, Cl− 181 pm) the sodium chloride
structure is adopted. Upon heating to above 450 °C, the normal caesium chloride structure (α-CsCl) converts to the β-CsCl form with the rocksalt structure (space group
Fmm).
s.
In contrast to sodium chloride
and potassium chloride
, caesium chloride readily dissolves in concentrated hydrochloric acid. Caesium chloride has also a relatively high solubility in formic acid
(1077 g/L at 18 °C) and hydrazine
; medium solubility in methanol
(31.7 g/L at 25 °C) and low solubility in ethanol
(7.6 g/L at 25 °C), sulfur dioxide
(2.95 g/L at 25 °C), ammonia
(3.8 g/L at 0 °C), acetone
(0.004% at 18 °С), acetonitrile
(0.083 g/L at 18 °С), ethylacetates and other complex ether
s, butanone
, acetophenone
, pyridine
and chlorobenzene
.
Despite its wide band gap
of about 8.35 eV at 80 K, caesium chloride weakly conducts electricity, and the conductivity is not electronic but ionic
. The conductivity has a value of the order 10−7 S/cm at 300 °C. It occurs through nearest-neighbor jumps of lattice vacancies, and the mobility is much higher for the Cl- than Cs+ vacancies. The conductivity increases with temperature up to about 450 °C, with an activation energy changing from 0.6 to 1.3 eV at about 260 °C. It then sharply drops by two orders of magnitude because of the phase transition from the α-CsCl to β-CsCl phase. The conductivity is also suppressed by application of pressure (about 10 times decrease at 0.4 GPa) which reduces the mobility of lattice vacancies.
in dilute solutions:

In aqueous solutions it enters common substitution reactions, for example:




CsCl converts to caesium sulfate
when boiled in concentrated sulfuric acid or heated with caesium hydrogen sulfate at 550–700 °С:
Caesium chloride forms a variety of double salts with other chlorides. Examples include 2CsCl·BaCl2, 2CsCl·CuCl2, CsCl·2CuCl and CsCl·LiCl, and with interhalogen
compounds:
In laboratory, CsCl can be obtained by treating caesium hydroxide
, carbonate
, caesium bicarbonate, or caesium sulfide with hydrochloric acid:
(KMgCl3·6H2O with up to 0.002% CsCl), sylvite
(KCl) and kainite
(MgSO4·KCl·3H2O), and in mineral waters. For example, the water of Bad Dürkheim
spa, which was used in isolation of caesium, contained about 0.17 mg/L of CsCl. None of these minerals are commercially important.
On industrial scale, CsCl is produced from the mineral pollucite
, which is powdered and treated with hydrochloric acid at elevated temperature. The extract is treated with antimony chloride
, iodine monochloride, or cerium(IV) chloride to give the poorly soluble double salt, e.g.:
Treatment of the double with hydrogen sulfide
gives CsCl:
High-purity CsCl is also produced from recrystallized Cs[ICl2] (and Cs[ICl4]) by thermal decomposition:
Only about 20 tonne
s of caesium compounds, with a major contribution from CsCl, were being produced annually around the 1970s and 2000s worldwide. Caesium chloride enriched with caesium-137 for radiation therapy
applications is produced at a single facility Mayak
in the Ural Region of Russia and is sold internationally through a UK dealer. The salt is synthesized at 200 °C because of its hygroscopic nature and sealed in a thimble-shaped steel container which is then enclosed into another steel casing. The sealing is required not only to reduce the radiation from the source, but also to protect the salt from moisture.
An analogous reaction – heating CsCl with calcium in vacuum in presence of phosphorus
was first reported in 1905 by the French chemist M. L. Hackspill and is still used industrially.
Caesium hydroxide
is obtained by electrolysis
of aqueous caesium chloride solution:
in a technique known as isopycnic centrifugation
. Centripetal and diffusive forces establish a density gradient that allow separation of mixtures on the basis of their molecular density. This technique allows separation of DNA of different densities (e.g. DNA fragments with differing A-T or G-C content). This application requires a solution with high density and yet relatively low viscosity, and CsCl suits it because of its high solubility in water, high density owing to the large mass of Cs, as well as low viscosity and high stability of CsCl solutions.
reagent in selected reactions. One of these reactions is the synthesis of glutamic acid
derivatives

where TBAB is tetrabutylammonium bromide (interphase catalyst) and CPME is a cyclopentyl methyl ether (solvent).
Another reaction is substitution of tetranitromethane

where DMF is dimethylformamide
(solvent).
used for detecting inorganic ions via the color and morphology of the precipitates. Quantitative concentration measurement of some of these ions, e.g. Mg2+, with inductively coupled plasma mass spectrometry, is used to evaluate the hardness of water.
! width="14%" |Ion
! width="20%" |Accompanying reagents
! Residue
! Morphology
! width="14%" |Detection limit (µg)
|-
| AsO33−
| KI
| Cs2[AsI5] or Cs3[AsI6]
| Red hexagons
| 0.01
|-
| Au3+
| AgCl, HCl
| Cs2Ag[AuCl6]
| Gray-black crosses, four and six-beamed stars
| 0.01
|-
| Au3+
| NH4SCN
| Cs[Au(SCN)4]
| Orange-yellow needles
| 0.4
|-
| Bi3+
| KI, HCl
| Cs2[BiI5] or 2.5H2O
| Red hexagons
| 0.13
|-
| Cu2+
| (CH3COO)2Pb, CH3COOH, KNO2
| Cs2Pb[Cu(NO2)6]
| Small black cubes
| 0.01
|-
| In3+
| —
| Cs3[InCl6]
| Small octahedra
| 0.02
|-
| [IrCl6]3−
| —
| Cs2[IrCl6]
| Small dark-red octahedra
| –
|-
| Mg2+
| Na2HPO4
| CsMgPO4 or 6H2O
| Small tetrahedra
| –
|-
| Pb2+
| KI
| Cs[PbI3]
| Yellow-green needles
| 0.01
|-
| Pd2+
| NaBr
| Cs2[PdBr4]
| Dark-red needles and prisms
| –
|-
| [ReCl4]−
| —
| Cs[ReCl4]
| Dark-red rhombs, bipyramids
| 0.2
|-
| [ReCl6]2−
| —
| Cs2[ReCl6]
| Small yellow-green octahedra
| 0.5
|-
| ReO4−
| —
| CsReO4
| Tetragonal bipyramids
| 0.13
|-
| Rh3+
| KNO2
| Cs3[Rh(NO2)6]
| Yellow cubes
| 0.1
|-
| Ru3+
| —
| Cs3[RuCl6]
| Pink needles
| –
|-
| [RuCl6]2−
| —
| Cs2[RuCl6]
| Small dark-red crystals
| 0.8
|-
| Sb3+
| —
| Cs2[SbCl5]·nH2O
| Hexagons
| 0.16
|-
| Sb3+
| NaI
| Cs[SbI4] or Cs2[SbI5]
| Red hexagons
| 0.1
|-
| Sn4+
| —
| Cs2[SnCl6]
| Small octahedra
| 0.2
|-
| TeO33−
| HCl
| Cs2[TeCl6]
| Light yellow octahedra
| 0.3
|-
| Tl3+
| NaI
| Cs[TlI4]
| Orange-red hexagons or rectangles
| 0.06
|}>
It is also used for detection of the following ions:
! width="14%" |Ion
! width="20%" |Accompanying reagents
! Detection
! width="14%" |Detection limit (µg/mL)
|-
| Al3+
| K2SO4
| Colorless crystals form in neutral media after evaporation
| 0.01
|-
| Ga3+
| KHSO4
| Colorless crystals form upon heating
| 0.5
|-
| Cr3+
| KHSO4
| Pale-violet crystals precipitate in slightly acidic media
| 0.06
|}>
and S. S. Botkin. They found that CsCl and RbCl induce long-term narrowing of the blood vessels (vasoconstriction
) and the associated increase in the blood pressure (hypertension
), stimulating the cardiovascular activity. These properties were then applied in the treatment of cardiovascular deceases.
Later research indicated that CsCl alleviates cardiac dysrhythmia
and that the life expectancy is higher in regions characterized by elevated levels of CsCl in water and food. Preliminary results indicate that CsCl can be used in the treatment of depressions. The neurological action of CsCl is related to the protection of neuron
s from apoptosis
and activation of caspase 3
caused by reduced potassium content.
Several reports suggested that non-radioactive caesium chloride can be used in a complex treatment of some forms of cancer. However, it has been linked to the deaths of over 50 patients, when it was used as part of a scientifically unvalidated cancer treatment. The American Cancer Society
considers caesium chloride therapy as requiring a further study for benefits and side effects.
, including treatment of cancer
(brachytherapy
) and diagnosis of myocardial infarction
. In the production of radioactive sources, it is normal to choose a chemical form of the radioisotope which would not be readily dispersed in the environment in the event of an accident. For instance, radiothermal generators (RTGs) often use strontium titanate
, which is insoluble in water. For teletherapy sources, however, the radioactive density (Ci in a given volume) needs to be very high, which is not possible with known insoluble caesium compounds. A thimble-shaped container of radioactive caesium chloride provides the active source.
es and screens of cathode ray tubes. It is a non-toxic provider of a halogen gas in exciplex lamps (exilamps) – a gas-discharge source of ultraviolet light which uses, for example, electrically excited XeCl molecules. Other uses include activation of electrodes in welding; manufacture of mineral water, beer and drilling muds; repellents and high-temperature solders. High-quality CsCl single crystals have a wide transparency range from UV to the infrared and therefore had been used for cuvettes, prisms and windows in optical spectrometers; this use was discontinued with the development of less hygroscopic materials.
s and cause asthma
.
Because of the high solubility in water, caesium chloride is highly mobile and can even diffuse through concrete. This is a drawback for its radioactive form which urges a search for more stable radioisotope materials. The commercial sources of radioactive caesium chloride are well sealed in a double steel enclosure. However, in the Goiânia accident
in Brazil
, such a source containing about 93 gram of 137CsCl, was stolen from an abandoned hospital and forced open by two scavengers. The blue glow emitted in the dark by the radioactive caesium chloride attracted the thieves and their relatives who were unaware of the associated dangers and spread the powder. This resulted in one of the worst radiation spill accidents in which 4 people died within a month from the exposure, 20 showed signs of radiation sickness
, 249 people were contaminated with radioactive caesium chloride, and about a thousand received a dose exceeding a yearly amount of background radiation. More than 110,000 people overwhelmed the local hospitals, and several city blocks had to be demolished in the cleanup operations. In the first days of the contamination, stomach disorders and nausea due to radiation sickness were experienced by several people, but only after several days one person associated the symptoms with the powder and brought a sample to the authorities.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
with the formula Cs
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
Cl
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
. This colorless solid is an important source of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s in a variety of applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chlorine ions. Caesium chloride crystals are thermally stable, but easily dissolve in water and concentrated hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, and therefore gradually disintegrate in the ambient conditions due to moisture. Caesium chloride occurs naturally in mineral waters and as an impurity in carnallite
Carnallite
Carnallite is an evaporite mineral, a hydrated potassium magnesium chloride with formula: KMgCl3·6. It is variably colored yellow to white, reddish, and sometimes colorless or blue. It is usually massive to fibrous with rare pseudohexagonal orthorhombic crystals...
(up to 0.002%), sylvite
Sylvite
Sylvite is potassium chloride in natural mineral form. It forms crystals in the isometric system very similar to normal rock salt, halite . The two are, in fact, isomorphous. Sylvite is colorless to white with shades of yellow and red due to inclusions. It has a Mohs hardness of 2.5 and a specific...
and kainite
Kainite
Kainite is a mineral salt that consists of potassium chloride and magnesium sulfate and is used as a fertilizer. This mineral is dull and soft, is colored white through yellow to red and is found in the Stassfurt salt mines in Saxony, Germany...
. Less than 20 tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite
Pollucite
Pollucite is a zeolite mineral with the formula 2Al2Si4O12·2H2O with iron, calcium, rubidium and potassium as common substituting elements. It is important as a significant ore of caesium and sometimes rubidium. It forms a solid solution series with analcime. It crystallizes in the isometric -...
.
Caesium chloride is widely used in isopycnic centrifugation
Isopycnic centrifugation
Isopycnic centrifugation, also known as density gradient centrifugation or equilibrium sedimentation is a technique used to separate molecules on the basis of buoyant density...
for separating various types of DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
. It is a reagent in analytical chemistry
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
, where it is used to identify ions by the color and morphology of the precipitate. When enriched in radioisotopes, such as 137CsCl or 131CsCl, caesium chloride is used in nuclear medicine
Nuclear medicine
In nuclear medicine procedures, elemental radionuclides are combined with other elements to form chemical compounds, or else combined with existing pharmaceutical compounds, to form radiopharmaceuticals. These radiopharmaceuticals, once administered to the patient, can localize to specific organs...
applications such as treatment of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
and diagnosis of myocardial infarction
Myocardial infarction
Myocardial infarction or acute myocardial infarction , commonly known as a heart attack, results from the interruption of blood supply to a part of the heart, causing heart cells to die...
. Another form of cancer treatment was studied using conventional non-radioactive CsCl. Whereas conventional caesium chloride has a rather low toxicity to humans and animals, the radioactive form easily contaminates the environment due to the high solubility of CsCl in water. Spread of 137CsCl powder from a 93-gram container in 1987 in Goiânia
Goiânia accident
The Goiânia accident was a radioactive contamination accident that occurred on September 13, 1987, at Goiânia, in the Brazilian State of Goiás after an old radiotherapy source was taken from an abandoned hospital site in the city...
, Brazil, resulted in one of the worst-ever radiation spill accidents killing four and directly affecting more than 100,000 people.
Crystal structure

Caesium bromide
Caesium bromide, , is an ionic compound of caesium and bromine. It has simple cubic p-type cubic crystallic structure, comparable to that of caesium chloride type with space group Pmm and lattice constant a = 0.42953 nm...
and CsI
Caesium iodide
Caesium iodide is an ionic compound often used as the input phosphor of an x-ray image intensifier tube found in fluoroscopy equipment....
and many binary metallic alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s. In contrast, the other alkaline halides have the sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
(rocksalt) structure. When both ions are similar in size (Cs+ ionic radius
Ionic radius
Ionic radius, rion, is the radius of an atom's ion. Although neither atoms nor ions have sharp boundaries, it is important to treat them as if they are hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice...
174 pm for this coordination number, Cl− 181 pm) the CsCl structure is adopted, when they are different (Na+ ionic radius
Ionic radius
Ionic radius, rion, is the radius of an atom's ion. Although neither atoms nor ions have sharp boundaries, it is important to treat them as if they are hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice...
102 pm, Cl− 181 pm) the sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
structure is adopted. Upon heating to above 450 °C, the normal caesium chloride structure (α-CsCl) converts to the β-CsCl form with the rocksalt structure (space group
Space group
In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct...
Fmm).
Physical properties
Caesium chloride is colorless in the form of large crystals and white when powdered. It readily dissolves in water with the maximum solubility increasing from 1865 g/L at 20 °C to 2705 g/L at 100 °C. The crystals are very hygroscopic and gradually disintegrate at ambient conditions. Caesium chloride does not form hydrateHydrate
Hydrate is a term used in inorganic chemistry and organic chemistry to indicate that a substance contains water. The chemical state of the water varies widely between hydrates, some of which were so labeled before their chemical structure was understood....
s.
In contrast to sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
and potassium chloride
Potassium chloride
The chemical compound potassium chloride is a metal halide salt composed of potassium and chlorine. In its pure state, it is odorless and has a white or colorless vitreous crystal appearance, with a crystal structure that cleaves easily in three directions. Potassium chloride crystals are...
, caesium chloride readily dissolves in concentrated hydrochloric acid. Caesium chloride has also a relatively high solubility in formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
(1077 g/L at 18 °C) and hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
; medium solubility in methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
(31.7 g/L at 25 °C) and low solubility in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
(7.6 g/L at 25 °C), sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
(2.95 g/L at 25 °C), ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
(3.8 g/L at 0 °C), acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
(0.004% at 18 °С), acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
(0.083 g/L at 18 °С), ethylacetates and other complex ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s, butanone
Butanone
Butanone, also known as methyl ethyl ketone or MEK, is an organic compound with the formula CH3CCH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of butterscotch and acetone. It is produced industrially on a large scale, and also occurs in trace amounts in nature...
, acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
and chlorobenzene
Chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.-Uses:...
.
Despite its wide band gap
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
of about 8.35 eV at 80 K, caesium chloride weakly conducts electricity, and the conductivity is not electronic but ionic
Ionic conductivity
Ionic conduction is the movement of an ion from one site to another through defects in the crystal lattice of a solid. Ionic conduction is one aspect of current....
. The conductivity has a value of the order 10−7 S/cm at 300 °C. It occurs through nearest-neighbor jumps of lattice vacancies, and the mobility is much higher for the Cl- than Cs+ vacancies. The conductivity increases with temperature up to about 450 °C, with an activation energy changing from 0.6 to 1.3 eV at about 260 °C. It then sharply drops by two orders of magnitude because of the phase transition from the α-CsCl to β-CsCl phase. The conductivity is also suppressed by application of pressure (about 10 times decrease at 0.4 GPa) which reduces the mobility of lattice vacancies.
Chemical properties
Caesium chloride completely dissociates upon dissolution in water, and the Cs+ cations are solvatedSolvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
in dilute solutions:

In aqueous solutions it enters common substitution reactions, for example:




CsCl converts to caesium sulfate
Caesium sulfate
Caesium sulfate is the caesium salt of sulfuric acid. It is used to prepare dense aqueous solutions for use in isopycnic centrifugation.-External links:*...
when boiled in concentrated sulfuric acid or heated with caesium hydrogen sulfate at 550–700 °С:
- 2 CsCl + H2SO4 → Cs2SO4 + 2 HCl
- CsCl + CsHSO4 → Cs2SO4 + HCl
Caesium chloride forms a variety of double salts with other chlorides. Examples include 2CsCl·BaCl2, 2CsCl·CuCl2, CsCl·2CuCl and CsCl·LiCl, and with interhalogen
Interhalogen
The halogens react with each other to form interhalogen compounds.The general formula of most interhalogen compounds is XYn, where n = 1, 3, 5 or 7, and X is the less electronegative of the two halogens...
compounds:
- CsCl + ICl3 → Cs[ICl4]
In laboratory, CsCl can be obtained by treating caesium hydroxide
Caesium hydroxide
Caesium hydroxide is a chemical compound consisting of an atom of caesium and a hydroxide group . It is a powerful base, much like other alkali metal hydroxides such as sodium hydroxide and potassium hydroxide...
, carbonate
Caesium carbonate
Caesium carbonate is a white crystalline solid of formula Cs2CO3. It is more soluble in organic solvents than many other carbonates such as potassium carbonate, and therefore finds use as a base in organic chemistry....
, caesium bicarbonate, or caesium sulfide with hydrochloric acid:
- CsOH + HCl → CsCl + H2O
- Cs2CO3 + 2 HCl → 2 CsCl + 2 H2O + CO2
Occurrence and production
Caesium chloride occurs naturally as an impurity in the halide minerals carnalliteCarnallite
Carnallite is an evaporite mineral, a hydrated potassium magnesium chloride with formula: KMgCl3·6. It is variably colored yellow to white, reddish, and sometimes colorless or blue. It is usually massive to fibrous with rare pseudohexagonal orthorhombic crystals...
(KMgCl3·6H2O with up to 0.002% CsCl), sylvite
Sylvite
Sylvite is potassium chloride in natural mineral form. It forms crystals in the isometric system very similar to normal rock salt, halite . The two are, in fact, isomorphous. Sylvite is colorless to white with shades of yellow and red due to inclusions. It has a Mohs hardness of 2.5 and a specific...
(KCl) and kainite
Kainite
Kainite is a mineral salt that consists of potassium chloride and magnesium sulfate and is used as a fertilizer. This mineral is dull and soft, is colored white through yellow to red and is found in the Stassfurt salt mines in Saxony, Germany...
(MgSO4·KCl·3H2O), and in mineral waters. For example, the water of Bad Dürkheim
Bad Dürkheim
Bad Dürkheim is a spa town in the Rhine-Neckar urban agglomeration, and is the seat of the Bad Dürkheim district in Rhineland-Palatinate, Germany.- Location :...
spa, which was used in isolation of caesium, contained about 0.17 mg/L of CsCl. None of these minerals are commercially important.
On industrial scale, CsCl is produced from the mineral pollucite
Pollucite
Pollucite is a zeolite mineral with the formula 2Al2Si4O12·2H2O with iron, calcium, rubidium and potassium as common substituting elements. It is important as a significant ore of caesium and sometimes rubidium. It forms a solid solution series with analcime. It crystallizes in the isometric -...
, which is powdered and treated with hydrochloric acid at elevated temperature. The extract is treated with antimony chloride
Antimony chloride
Antimony chloride may refer to either of the following:*Antimony trichloride, SbCl3*Antimony pentachloride, SbCl5...
, iodine monochloride, or cerium(IV) chloride to give the poorly soluble double salt, e.g.:
- CsCl + SbCl3 → CsSbCl4
Treatment of the double with hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
gives CsCl:
- 2 CsSbCl4 + 3 H2S → 2 CsCl + Sb2S3 + 8 HCl
High-purity CsCl is also produced from recrystallized Cs[ICl2] (and Cs[ICl4]) by thermal decomposition:
- Cs[ICl2] → 2 CsCl + ICl
Only about 20 tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s of caesium compounds, with a major contribution from CsCl, were being produced annually around the 1970s and 2000s worldwide. Caesium chloride enriched with caesium-137 for radiation therapy
Radiation therapy
Radiation therapy , radiation oncology, or radiotherapy , sometimes abbreviated to XRT or DXT, is the medical use of ionizing radiation, generally as part of cancer treatment to control malignant cells.Radiation therapy is commonly applied to the cancerous tumor because of its ability to control...
applications is produced at a single facility Mayak
Mayak
Mayak Production Association refers to an industrial complex that is one of the biggest nuclear facilities in the Russian Federation. It housed plutonium production reactors and a reprocessing plant...
in the Ural Region of Russia and is sold internationally through a UK dealer. The salt is synthesized at 200 °C because of its hygroscopic nature and sealed in a thimble-shaped steel container which is then enclosed into another steel casing. The sealing is required not only to reduce the radiation from the source, but also to protect the salt from moisture.
Precursor to Cs metal
Caesium chloride is the main precursor to caesium metal by high temperature reduction:- 2 CsCl + Mg → MgCl2 + Cs
An analogous reaction – heating CsCl with calcium in vacuum in presence of phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
was first reported in 1905 by the French chemist M. L. Hackspill and is still used industrially.
Caesium hydroxide
Caesium hydroxide
Caesium hydroxide is a chemical compound consisting of an atom of caesium and a hydroxide group . It is a powerful base, much like other alkali metal hydroxides such as sodium hydroxide and potassium hydroxide...
is obtained by electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of aqueous caesium chloride solution:
- 2 CsCl + 2 H2O → 2 CsOH + Cl2 + H2
Solute for ultracentrifugation
Caesium chloride is widely used in centrifugationCentrifugation
Centrifugation is a process that involves the use of the centrifugal force for the sedimentation of mixtures with a centrifuge, used in industry and in laboratory settings. More-dense components of the mixture migrate away from the axis of the centrifuge, while less-dense components of the mixture...
in a technique known as isopycnic centrifugation
Isopycnic centrifugation
Isopycnic centrifugation, also known as density gradient centrifugation or equilibrium sedimentation is a technique used to separate molecules on the basis of buoyant density...
. Centripetal and diffusive forces establish a density gradient that allow separation of mixtures on the basis of their molecular density. This technique allows separation of DNA of different densities (e.g. DNA fragments with differing A-T or G-C content). This application requires a solution with high density and yet relatively low viscosity, and CsCl suits it because of its high solubility in water, high density owing to the large mass of Cs, as well as low viscosity and high stability of CsCl solutions.
Organic chemistry
Caesium chloride is rarely used in organic chemistry. It can act as a phase transfer catalystPhase transfer catalyst
In chemistry, a phase transfer catalyst or PTC is a catalyst that facilitates the migration of a reactant from one phase into another phase where reaction occurs. Phase transfer catalysis is a special form of heterogeneous catalysis. Ionic reactants are often soluble in an aqueous phase but...
reagent in selected reactions. One of these reactions is the synthesis of glutamic acid
Glutamic acid
Glutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates...
derivatives

where TBAB is tetrabutylammonium bromide (interphase catalyst) and CPME is a cyclopentyl methyl ether (solvent).
Another reaction is substitution of tetranitromethane
Tetranitromethane
Tetranitromethane or TNM is an organic oxidizer with chemical formula C4. Its chemical structure consists of four nitro groups attached to one carbon atom...

where DMF is dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
(solvent).
Analytical chemistry
Caesium chloride is a reagent in traditional analytical chemistryAnalytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
used for detecting inorganic ions via the color and morphology of the precipitates. Quantitative concentration measurement of some of these ions, e.g. Mg2+, with inductively coupled plasma mass spectrometry, is used to evaluate the hardness of water.
! width="20%" |Accompanying reagents
! Residue
! Morphology
! width="14%" |Detection limit (µg)
|-
| AsO33−
| KI
| Cs2[AsI5] or Cs3[AsI6]
| Red hexagons
| 0.01
|-
| Au3+
| AgCl, HCl
| Cs2Ag[AuCl6]
| Gray-black crosses, four and six-beamed stars
| 0.01
|-
| Au3+
| NH4SCN
| Cs[Au(SCN)4]
| Orange-yellow needles
| 0.4
|-
| Bi3+
| KI, HCl
| Cs2[BiI5] or 2.5H2O
| Red hexagons
| 0.13
|-
| Cu2+
| (CH3COO)2Pb, CH3COOH, KNO2
| Cs2Pb[Cu(NO2)6]
| Small black cubes
| 0.01
|-
| In3+
| —
| Cs3[InCl6]
| Small octahedra
| 0.02
|-
| [IrCl6]3−
| —
| Cs2[IrCl6]
| Small dark-red octahedra
| –
|-
| Mg2+
| Na2HPO4
| CsMgPO4 or 6H2O
| Small tetrahedra
| –
|-
| Pb2+
| KI
| Cs[PbI3]
| Yellow-green needles
| 0.01
|-
| Pd2+
| NaBr
| Cs2[PdBr4]
| Dark-red needles and prisms
| –
|-
| [ReCl4]−
| —
| Cs[ReCl4]
| Dark-red rhombs, bipyramids
| 0.2
|-
| [ReCl6]2−
| —
| Cs2[ReCl6]
| Small yellow-green octahedra
| 0.5
|-
| ReO4−
| —
| CsReO4
| Tetragonal bipyramids
| 0.13
|-
| Rh3+
| KNO2
| Cs3[Rh(NO2)6]
| Yellow cubes
| 0.1
|-
| Ru3+
| —
| Cs3[RuCl6]
| Pink needles
| –
|-
| [RuCl6]2−
| —
| Cs2[RuCl6]
| Small dark-red crystals
| 0.8
|-
| Sb3+
| —
| Cs2[SbCl5]·nH2O
| Hexagons
| 0.16
|-
| Sb3+
| NaI
| Cs[SbI4] or Cs2[SbI5]
| Red hexagons
| 0.1
|-
| Sn4+
| —
| Cs2[SnCl6]
| Small octahedra
| 0.2
|-
| TeO33−
| HCl
| Cs2[TeCl6]
| Light yellow octahedra
| 0.3
|-
| Tl3+
| NaI
| Cs[TlI4]
| Orange-red hexagons or rectangles
| 0.06
|}>
It is also used for detection of the following ions:
! width="20%" |Accompanying reagents
! Detection
! width="14%" |Detection limit (µg/mL)
|-
| Al3+
| K2SO4
| Colorless crystals form in neutral media after evaporation
| 0.01
|-
| Ga3+
| KHSO4
| Colorless crystals form upon heating
| 0.5
|-
| Cr3+
| KHSO4
| Pale-violet crystals precipitate in slightly acidic media
| 0.06
|}>
Medicine
Medical properties of caesium chloride were studied back in 1888 by Ivan PavlovIvan Pavlov
Ivan Petrovich Pavlov was a famous Russian physiologist. Although he made significant contributions to psychology, he was not in fact a psychologist himself but was a mathematician and actually had strong distaste for the field....
and S. S. Botkin. They found that CsCl and RbCl induce long-term narrowing of the blood vessels (vasoconstriction
Vasoconstriction
Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, particularly the large arteries, small arterioles and veins. The process is the opposite of vasodilation, the widening of blood vessels. The process is particularly important in...
) and the associated increase in the blood pressure (hypertension
Hypertension
Hypertension or high blood pressure is a cardiac chronic medical condition in which the systemic arterial blood pressure is elevated. What that means is that the heart is having to work harder than it should to pump the blood around the body. Blood pressure involves two measurements, systolic and...
), stimulating the cardiovascular activity. These properties were then applied in the treatment of cardiovascular deceases.
Later research indicated that CsCl alleviates cardiac dysrhythmia
Cardiac dysrhythmia
Cardiac dysrhythmia is any of a large and heterogeneous group of conditions in which there is abnormal electrical activity in the heart. The heart beat may be too fast or too slow, and may be regular or irregular.Some arrhythmias are life-threatening medical emergencies that can result in cardiac...
and that the life expectancy is higher in regions characterized by elevated levels of CsCl in water and food. Preliminary results indicate that CsCl can be used in the treatment of depressions. The neurological action of CsCl is related to the protection of neuron
Neuron
A neuron is an electrically excitable cell that processes and transmits information by electrical and chemical signaling. Chemical signaling occurs via synapses, specialized connections with other cells. Neurons connect to each other to form networks. Neurons are the core components of the nervous...
s from apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
and activation of caspase 3
Caspase 3
Caspase 3 is a caspase protein that interacts with caspase 8 and caspase 9. It is encoded by the CASP3 gene. CASP3 orthologs have been identified in numerous mammals for which complete genome data are available...
caused by reduced potassium content.
Several reports suggested that non-radioactive caesium chloride can be used in a complex treatment of some forms of cancer. However, it has been linked to the deaths of over 50 patients, when it was used as part of a scientifically unvalidated cancer treatment. The American Cancer Society
American Cancer Society
The American Cancer Society is the "nationwide community-based voluntary health organization" dedicated, in their own words, "to eliminating cancer as a major health problem by preventing cancer, saving lives, and diminishing suffering from cancer, through research, education, advocacy, and...
considers caesium chloride therapy as requiring a further study for benefits and side effects.
Nuclear medicine and radiography
Caesium chloride composed of radioisotopes such as 137CsCl and 131CsCl, is used in nuclear medicineNuclear medicine
In nuclear medicine procedures, elemental radionuclides are combined with other elements to form chemical compounds, or else combined with existing pharmaceutical compounds, to form radiopharmaceuticals. These radiopharmaceuticals, once administered to the patient, can localize to specific organs...
, including treatment of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
(brachytherapy
Brachytherapy
Brachytherapy , also known as internal radiotherapy, sealed source radiotherapy, curietherapy or endocurietherapy, is a form of radiotherapy where a radiation source is placed inside or next to the area requiring treatment...
) and diagnosis of myocardial infarction
Myocardial infarction
Myocardial infarction or acute myocardial infarction , commonly known as a heart attack, results from the interruption of blood supply to a part of the heart, causing heart cells to die...
. In the production of radioactive sources, it is normal to choose a chemical form of the radioisotope which would not be readily dispersed in the environment in the event of an accident. For instance, radiothermal generators (RTGs) often use strontium titanate
Strontium titanate
Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure...
, which is insoluble in water. For teletherapy sources, however, the radioactive density (Ci in a given volume) needs to be very high, which is not possible with known insoluble caesium compounds. A thimble-shaped container of radioactive caesium chloride provides the active source.
Miscellaneous applications
Caesium chloride is used in the preparation of electrically conducting glassGlass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
es and screens of cathode ray tubes. It is a non-toxic provider of a halogen gas in exciplex lamps (exilamps) – a gas-discharge source of ultraviolet light which uses, for example, electrically excited XeCl molecules. Other uses include activation of electrodes in welding; manufacture of mineral water, beer and drilling muds; repellents and high-temperature solders. High-quality CsCl single crystals have a wide transparency range from UV to the infrared and therefore had been used for cuvettes, prisms and windows in optical spectrometers; this use was discontinued with the development of less hygroscopic materials.
Toxicity
Caesium chloride has a low toxicity to human and animals. Its median lethal dose (LD50) in mice is 2300 mg per kilogram of body weight for oral administration and 910 mg/kg for intravenous injection. The toxicity of CsCl is related to its ability to lower the concentration of potassium in the body and partly substitute it in biochemical processes. Caesium chloride powder can irritate the mucous membraneMucous membrane
The mucous membranes are linings of mostly endodermal origin, covered in epithelium, which are involved in absorption and secretion. They line cavities that are exposed to the external environment and internal organs...
s and cause asthma
Asthma
Asthma is the common chronic inflammatory disease of the airways characterized by variable and recurring symptoms, reversible airflow obstruction, and bronchospasm. Symptoms include wheezing, coughing, chest tightness, and shortness of breath...
.
Because of the high solubility in water, caesium chloride is highly mobile and can even diffuse through concrete. This is a drawback for its radioactive form which urges a search for more stable radioisotope materials. The commercial sources of radioactive caesium chloride are well sealed in a double steel enclosure. However, in the Goiânia accident
Goiânia accident
The Goiânia accident was a radioactive contamination accident that occurred on September 13, 1987, at Goiânia, in the Brazilian State of Goiás after an old radiotherapy source was taken from an abandoned hospital site in the city...
in Brazil
Brazil
Brazil , officially the Federative Republic of Brazil , is the largest country in South America. It is the world's fifth largest country, both by geographical area and by population with over 192 million people...
, such a source containing about 93 gram of 137CsCl, was stolen from an abandoned hospital and forced open by two scavengers. The blue glow emitted in the dark by the radioactive caesium chloride attracted the thieves and their relatives who were unaware of the associated dangers and spread the powder. This resulted in one of the worst radiation spill accidents in which 4 people died within a month from the exposure, 20 showed signs of radiation sickness
Radiation Sickness
Radiation Sickness is a VHS by the thrash metal band Nuclear Assault. The video is a recording of a concert at the Hammersmith Odeon, London in 1988. It was released in 1991...
, 249 people were contaminated with radioactive caesium chloride, and about a thousand received a dose exceeding a yearly amount of background radiation. More than 110,000 people overwhelmed the local hospitals, and several city blocks had to be demolished in the cleanup operations. In the first days of the contamination, stomach disorders and nausea due to radiation sickness were experienced by several people, but only after several days one person associated the symptoms with the powder and brought a sample to the authorities.

