Covalent superconductors
Encyclopedia
Covalent semiconductor
s are such solids as diamond
, silicon
, germanium
, silicon carbide
and silicon-germanium where atoms are linked by covalent bond
s. Most of those materials, at least in their bulk form, are well studied and rarely hit the front pages of the top scientific journals in the last decade. However, issue 23 of volume 93 (2004) of a major physics journal Physical Review Letters
contained as many as 4 papers on diamond. Those papers were a reaction to a breakthrough discovery of superconductivity
in synthetic diamond
grown by high-pressure high-temperature (HPHT) method. The discovery had no practical importance but surprised most scientists as superconductivity
has not been considered seriously in covalent semiconductors.
by boron such that the individual doping atoms started interacting and formed an "impurity band". The superconductivity was of type-II with the critical temperature Tc = 4 K and critical magnetic field Hc = 4 T. Later, Tc ~ 11K has been achieved in homoepitaxial CVD
films.
Regarding the origin of superconductivity in diamond, three alternative theories exist at the moment: conventional BCS theory
based on phonon-mediated pairing, correlated impurity band theory and spin-flip-driven pairing of holes weakly localized in the vicinity of the Fermi level. Whereas there is no solid experimental support for either model, recent accurate measurements of isotopic shift of the transition temperature Tc upon boron and carbon isotopic substitutions favor the BCS theory.
. In attempt to explain this difference, it was noted that Si sites are more important than carbon sites for superconductivity in SiC. Whereas boron substitutes carbon in SiC, Al substitutes Si sites. Therefore, Al and B "see" different environment that might explain different properties of SiC:Al and SiC:B.
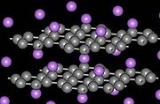
 When metal atoms are inserted (intercalated) between the graphite planes, several superconductors are created with the following transition temperatures:
When metal atoms are inserted (intercalated) between the graphite planes, several superconductors are created with the following transition temperatures:
). Another example, after Sumio Iijima
has "discovered" carbon nanotube
s in 1991, many scientists have pointed out that carbon nanofibers were actually observed decades earlier. The same could be said about superconductivity in covalent semiconductors. Superconductivity in germanium
and silicon-germanium was predicted theoretically as early as in the 1960s. Shortly after, superconductivity was experimentally detected in germanium telluride
. In 1976, superconductivity with Tc = 3.5 K was observed experimentally in germanium
implanted with copper ions; it was experimentally demonstrated that amorphization was essential for the superconductivity (in Ge), and the superconductivity was assigned to Ge itself, not copper.
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
s are such solids as diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
, silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
, silicon carbide
Silicon carbide
Silicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
and silicon-germanium where atoms are linked by covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s. Most of those materials, at least in their bulk form, are well studied and rarely hit the front pages of the top scientific journals in the last decade. However, issue 23 of volume 93 (2004) of a major physics journal Physical Review Letters
Physical Review Letters
Physical Review Letters , established in 1958, is a peer reviewed, scientific journal that is published 52 times per year by the American Physical Society...
contained as many as 4 papers on diamond. Those papers were a reaction to a breakthrough discovery of superconductivity
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
in synthetic diamond
Synthetic diamond
Synthetic diamond is diamond produced in a technological process; as opposed to natural diamond, which is created in geological processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond, denoting the production method, High-Pressure High-Temperature synthesis and Chemical...
grown by high-pressure high-temperature (HPHT) method. The discovery had no practical importance but surprised most scientists as superconductivity
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
has not been considered seriously in covalent semiconductors.
Diamond
Superconductivity in diamond was achieved through heavy p-type dopingP-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
by boron such that the individual doping atoms started interacting and formed an "impurity band". The superconductivity was of type-II with the critical temperature Tc = 4 K and critical magnetic field Hc = 4 T. Later, Tc ~ 11K has been achieved in homoepitaxial CVD
Chemical vapor deposition of diamond
Chemical vapor deposition of diamond or CVD is a method of producing synthetic diamond by creating the circumstances necessary for carbon atoms in a gas to settle on a substrate in crystalline form....
films.
Regarding the origin of superconductivity in diamond, three alternative theories exist at the moment: conventional BCS theory
BCS theory
BCS theory — proposed by Bardeen, Cooper, and Schrieffer in 1957 — is the first microscopic theory of superconductivity since its discovery in 1911. The theory describes superconductivity as a microscopic effect caused by a "condensation" of pairs of electrons into a boson-like state...
based on phonon-mediated pairing, correlated impurity band theory and spin-flip-driven pairing of holes weakly localized in the vicinity of the Fermi level. Whereas there is no solid experimental support for either model, recent accurate measurements of isotopic shift of the transition temperature Tc upon boron and carbon isotopic substitutions favor the BCS theory.
Silicon
It was suggested that "Si and Ge, which also form in the diamond structure, may similarly exhibit superconductivity under the appropriate conditions", and indeed, discoveries of superconductivity in heavily boron doped Si (Si:B) and SiC:B have quickly followed. Similar to diamond, Si:B is type-II superconductor, but it has much smaller values of Tc = 0.4 K and Hc = 0.4 T. Superconductivity in Si:B was achieved by heavy doping (above 8 at.%), realized through a special non-equilibrium technique of gas immersion laser doping.Silicon carbide
Superconductivity in SiC was achieved by heavy doping with boron or aluminum. Both the cubic (3C-SiC) and hexagonal (6H-SiC) phases are superconducting and show a very similar Tc of 1.5 K. A crucial difference is however observed for the magnetic field behavior between aluminum and boron doping: SiC:Al is type-II, same as Si:B. On the contrary, SiC:B is type-IType I superconductor
Superconductors cannot be penetrated by magnetic flux lines . This Meissner state breaks down when the applied magnetic field is too large. Superconductors can be divided into two classes according to how this breakdown occurs...
. In attempt to explain this difference, it was noted that Si sites are more important than carbon sites for superconductivity in SiC. Whereas boron substitutes carbon in SiC, Al substitutes Si sites. Therefore, Al and B "see" different environment that might explain different properties of SiC:Al and SiC:B.
Carbon nanotubes
Superconductivity in carbon nanotubes has been observed experimentally in 2001. Note however a crucial difference between nanotubes and diamond: Although nanotubes contain covalently bonded carbon atoms, they are closer in properties to graphite than diamond, and can be metallic without doping. Meanwhile, undoped diamond is an insulator.Intercalated graphite

| Material | CaC6 | Li3Ca2C6 | YbC6 | SrC6 | KC8 | RbC8 | NaC3 | KC3 | LiC3 | NaC2 | LiC2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tc (K) | 11.5 | 11.15 | 6.5 | 1.65 | 0.14 | 0.025 | 2.3-3.8 | 3.0 | <0.35 | 5.0 | 1.9 |
History
The priority of many discoveries in science is vigorously disputed (see, e.g., Nobel Prize controversiesNobel Prize controversies
Subsequent to his death in 1896, the will of Swedish industrialist Alfred Nobel established the Nobel Prizes. Annual prizes were to be awarded for service to humanity in the fields of physics, chemistry, physiology or medicine, literature, and peace. Similarly, the Bank of Sweden Prize in Economic...
). Another example, after Sumio Iijima
Sumio Iijima
Sumio Iijima is a Japanese physicist, often cited as the discoverer of carbon nanotubes. Although carbon nanotubes had been observed prior to his "discovery", Iijima's 1991 paper generated unprecedented interest in the carbon nanostructures and has since fueled intense research in the area of...
has "discovered" carbon nanotube
Carbon nanotube
Carbon nanotubes are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1, significantly larger than for any other material...
s in 1991, many scientists have pointed out that carbon nanofibers were actually observed decades earlier. The same could be said about superconductivity in covalent semiconductors. Superconductivity in germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
and silicon-germanium was predicted theoretically as early as in the 1960s. Shortly after, superconductivity was experimentally detected in germanium telluride
Germanium telluride
Germanium telluride is a chemical compound of germanium and tellurium and is a component of chalcogenide glasses. It shows semimetallic conduction and ferroelectric behaviour....
. In 1976, superconductivity with Tc = 3.5 K was observed experimentally in germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
implanted with copper ions; it was experimentally demonstrated that amorphization was essential for the superconductivity (in Ge), and the superconductivity was assigned to Ge itself, not copper.
See also
- Conventional superconductorConventional superconductorConventional superconductors are materials that display superconductivity as described by BCS theory or its extensions.Critical temperatures of some simple metals:ElementTc Al1.20Hg4.15Mo0.92Nb9.26Pb7.19...
- List of superconductors
- High-temperature superconductivityHigh-temperature superconductivityHigh-temperature superconductors are materials that have a superconducting transition temperature above . From 1960 to 1980, 30 K was thought to be the highest theoretically possible Tc...
- Room temperature superconductorRoom temperature superconductorA room-temperature superconductor is a material yet to be discovered which would be capable of exhibiting superconducting properties at operating temperatures above 0° C . This is not strictly speaking "room temperature" A room-temperature superconductor is a material yet to be discovered...
- SuperconductivitySuperconductivitySuperconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
- Superconductor classificationSuperconductor classificationSuperconductors can be classified in accordance with several criteria that depend on our interest in their physical properties, on the understanding we have about them, on how expensive is cooling them or on the material they are made of....
- Technological applications of superconductivityTechnological applications of superconductivitySome of the technological applications of superconductivity include:* the production of sensitive magnetometers based on SQUIDs* fast digital circuits ,...
- Timeline of low-temperature technologyTimeline of low-temperature technologyThe following is a timeline of low-temperature technology and cryogenic technology .-16th century BCE – 17th century CE :...
- Type-I superconductor
- Type-II superconductorType-II superconductorA Type-II superconductor is a superconductor characterized by the formation of vortex lattices in magnetic field. It has a continuous second order phase transition from the superconducting to the normal state within an increasing magnetic field....
- Unconventional superconductorUnconventional superconductorUnconventional superconductors are materials that display superconductivity which does not conform to either the conventional BCS theory or the Nikolay Bogolyubov's theory or its extensions....
- SiliconSiliconSilicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
- Silicon carbideSilicon carbideSilicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
- Synthetic diamondSynthetic diamondSynthetic diamond is diamond produced in a technological process; as opposed to natural diamond, which is created in geological processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond, denoting the production method, High-Pressure High-Temperature synthesis and Chemical...
Free-download papers
- International Workshop on superconductivity in Diamond and Related Materials 2005
- International Workshop on Superconductivity in Diamond and Related Materials 2008
- New Diamond and Frontier Carbon Technology Volume 17, No.1 Special Issue on Superconductivity in CVD Diamond
- Some papers on superconducting diamond

