
Free radical reaction
Encyclopedia
A free radical reaction is any chemical reaction
involving free radicals. This reaction type is abundant in organic reaction
s.
Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical
by Moses Gomberg
(1900) and the lead-mirror experiment described by Friedrich Paneth
in 1927. In this last experiment tetramethyllead is decomposed
at elevated temperatures to methyl radicals and elemental lead in a quartz
tube. The gaseous methyl radicals are moved to another part of the chamber in a carrier gas where they react with lead in a mirror film which slowly disappears.
When radical reactions are part of organic synthesis
the radicals are often generated from radical initiator
s such as peroxides or azobis compounds. Many radical reactions are chain reactions with a chain initiation step, a chain propagation
step and a chain termination
step. Reaction inhibitor
s slow down a radical reaction and radical disproportionation
is a competing reaction. Radical reactions occur frequently in the gas phase, are often initiated by light, are rarely acid or base catalyzed an are not dependent on polarity of the reaction medium. Reactions are also similar whether in the gas phase or solution phase.
of a radical reaction depend on all these individual reactions. In steady state
the concentrations of initiating (I.) and terminating species T. are negligent and rate of initiation and rate of termination are equal. The overall reaction rate
can be written as :
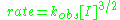
with a broken-order dependence of 1.5 with respect to the initiating species.
The reactivity of different compounds toward a certain radical is measured in so-called competition experiments. Compounds bearing carbon-hydrogen bonds react with radicals in the order primary < secondary < tertiary < benzyl
< allyl
reflecting the order in C-H bond dissociation energy
Many stabilizing effects can be explained as resonance effects, an effect specific to radicals is the captodative effect
.
it can be formed by photochemical reaction and thermal fission reaction or by oxidation reduction reaction
Specific reactions involving free radicals are combustion
, pyrolysis
and cracking
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
involving free radicals. This reaction type is abundant in organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s.
Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical
Triphenylmethyl radical
The triphenylmethyl radical is a persistent radical and the first-ever radical described in organic chemistry. It can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type...
by Moses Gomberg
Moses Gomberg
Moses Gomberg was a chemistry professor at the University of Michigan....
(1900) and the lead-mirror experiment described by Friedrich Paneth
Friedrich Paneth
Friedrich Adolf Paneth was an Austrian-born British chemist. Fleeing the Nazis, he escaped to Britain and became a British citizen in 1939 but returned as director of the Max Planck Institute for Chemistry in 1953....
in 1927. In this last experiment tetramethyllead is decomposed
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
at elevated temperatures to methyl radicals and elemental lead in a quartz
Quartz
Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,...
tube. The gaseous methyl radicals are moved to another part of the chamber in a carrier gas where they react with lead in a mirror film which slowly disappears.
When radical reactions are part of organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
the radicals are often generated from radical initiator
Radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
s such as peroxides or azobis compounds. Many radical reactions are chain reactions with a chain initiation step, a chain propagation
Chain propagation
Chain propagation is a process in which a reactive intermediate is continuously regenerated during the course of a chemical reaction. In polymerization reaction, the reactive end-groups of a polymer chain react in each propagation step with a new monomer molecule transferring the reactive group to...
step and a chain termination
Chain termination
Chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.- Mechanisms of Termination :...
step. Reaction inhibitor
Reaction inhibitor
A reaction inhibitor is a substance that decreases the rate of, or prevents, a chemical reaction.-Inhibition of a catalyst:An inhibitor can reduce the effectiveness of a catalyst in a catalysed reaction...
s slow down a radical reaction and radical disproportionation
Radical disproportionation
Radicals in chemistry are defined as reactive atoms or molecules that contain unpaired electrons in an open shell. The unpaired electrons cause radicals to be unstable and reactive. Reactions in radical chemistry can generate both radical and non-radical products...
is a competing reaction. Radical reactions occur frequently in the gas phase, are often initiated by light, are rarely acid or base catalyzed an are not dependent on polarity of the reaction medium. Reactions are also similar whether in the gas phase or solution phase.
Kinetics
The chemical kineticsChemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
of a radical reaction depend on all these individual reactions. In steady state
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
the concentrations of initiating (I.) and terminating species T. are negligent and rate of initiation and rate of termination are equal. The overall reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
can be written as :
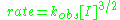
with a broken-order dependence of 1.5 with respect to the initiating species.
The reactivity of different compounds toward a certain radical is measured in so-called competition experiments. Compounds bearing carbon-hydrogen bonds react with radicals in the order primary < secondary < tertiary < benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
< allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
reflecting the order in C-H bond dissociation energy
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
Many stabilizing effects can be explained as resonance effects, an effect specific to radicals is the captodative effect
Captodative effect
The captodative effect in chemistry is the effect on the stability of a carbon-centred radical determined by the combined action of a captor and a dative substituent, both attached to the radical centre...
.
Reactions
The most important reaction types involving free radicals are:- Free radical substitution, for instance free radical halogenationFree radical halogenationIn organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of heat or UV light. The reaction is used for the industrial synthesis of chloroform , dichloromethane , and hexachlorobutadiene...
and autoxidationAutoxidationAutoxidation is any oxidation that occurs in open air or in presence of oxygen and/or UV radiation and forms peroxides and hydroperoxides. A classic example of autoxidation is that of simple ethers like diethyl ether, whose peroxides can be dangerously explosive. It can be considered to be a slow,...
. - Free radical additionFree radical additionFree radical addition is an addition reaction in organic chemistry involving free radicals. The addition may occur between a radical and a non-radical, or between two radicals....
reactions - IntramolecularIntramolecularIntramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
free radical reactions (substitution or addition) such as the Hofmann-Löffler reactionHofmann-Löffler reactionThe Hofmann-Löffler reaction is an organic reaction in which a cyclic amine 2 is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid...
or the Barton reactionBarton reactionThe Barton Reaction involves the photolysis of a nitrite to form a δ-nitroso alcohol. It is named for the British chemist Sir Derek Harold Richard Barton... - Free radical rearrangement reactionRearrangement reactionA rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
s are rare compared to rearrangements involving carbocations and restricted to arylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
migrations. - Fragmentation reactions or homolysisHomolysisIn general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
, for instance the Norrish reactionNorrish reactionThe Norrish reaction in organic chemistry describes the photochemical reactions taking place with ketones and aldehydes. This type of reaction is subdivided in Norrish type I reactions and Norrish type II reactions...
, the Hunsdiecker reactionHunsdiecker reactionThe Hunsdiecker reaction is the organic reaction of silver salts of carboxylic acids with halogens to give organic halides. It is an example of a halogenation reaction...
and certain decarboxylationDecarboxylationDecarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
s. For fragmentations taking place in mass spectrometry see mass spectrum analysisMass spectrum analysisMass-spectrum analysis is an integral part of mass spectrometry. Organic chemists obtain mass spectra of chemical compounds as part of structure elucidation and the analysis is part of every organic chemistry curriculum.-Basic peaks:...
. - Electron transferElectron transferElectron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
. An example is the decomposition of certain peresters by Cu(I)CopperCopper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
which is a one-electron reductionOne-electron reductionA one-electron reduction in organic chemistry involves the transfer of an electron from a metal to an organic substrate. It serves to differentiate between true organic reductions and other reductions such as hydride transfer reactions that actually involve two-electron species.The first...
reaction forming Cu(II), an alkoxy oxygen radical and a carboxylate. Another example is Kolbe electrolysisKolbe electrolysisKolbe electrolysis or Kolbe reaction is an organic reaction named after Adolph Wilhelm Hermann Kolbe. The Kolbe reaction is formally a decarboxylative dimerisation and proceeds by a radical reaction mechanism...
. - Radical-nucleophilic aromatic substitutionRadical-nucleophilic aromatic substitutionRadical-nucleophilic aromatic substitution or SRN1 in organic chemistry is a type of substitution reaction in which a certain substituent on an aromatic compound is replaced by a nucleophile through an intermediary free radical species:...
is a special case of nucleophilic aromatic substitutionNucleophilic aromatic substitutionright|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
.
it can be formed by photochemical reaction and thermal fission reaction or by oxidation reduction reaction
Specific reactions involving free radicals are combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
, pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
and cracking
Cracking (chemistry)
In petroleum geology and chemistry, cracking is the process whereby complex organic molecules such as kerogens or heavy hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products...

