One-electron reduction
Encyclopedia
A one-electron reduction in organic chemistry
involves the transfer of an electron from a metal
to an organic
substrate
. It serves to differentiate between true organic reductions and other reductions such as hydride
transfer reactions that actually involve two-electron species.
The first intermediate in a one-electron reduction is often a radical anion
, which then engages in secondary reactions. In the Birch reduction
, the secondary reaction is proton abstraction from an alcohol
. This reaction type is also called a dissolving metal reduction. Alkyne
reduction to an alkene
in the liquid ammonia
/sodium
system follows the same theme. The first radical anion intermediate abstracts a proton from ammonia
to the free radical. A second one-electron transfer leads to the anion, which also abstracts a proton to the neutral alkene.
In the Wurtz reaction
, two radical intermediates dimerize in a coupling reaction
. Likewise, acetone
is converted to pinacol
with a magnesium-mercury amalgam
in a pinacol coupling reaction. Acyloin condensation
couples two carboxylic acid
s to a α-hydroxyketone. Reactions of this type are also called reductive couplings. In the Clemmensen reduction
of ketones to alkanes with zinc-mercury amalgam, the intermediate is an organozinc carbenoid.
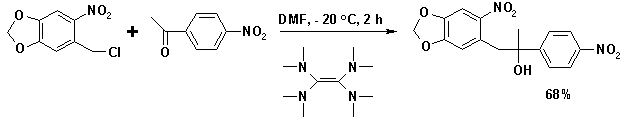
Electron rich organic molecules like tetrakis(dimethylamino)ethylene (TDAE) are effective reducing agents capable of generating the anion from alkyl halides such as 5-chloromethyl-6-nitrobenzo[1,3]dioxole:
The one-electron reduction potential of a molecule can be used to obtain an electron affinity. For example: The one-electron reduction potential of molecular oxygen gives a value of 1.07(1) eV.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
involves the transfer of an electron from a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
to an organic
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
. It serves to differentiate between true organic reductions and other reductions such as hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
transfer reactions that actually involve two-electron species.
The first intermediate in a one-electron reduction is often a radical anion
Radical ion
A radical ion is a free radical species that carries a charge. Radical ions are encountered in organic chemistry as reactive intermediates and in mass spectrometry as gas phase ions...
, which then engages in secondary reactions. In the Birch reduction
Birch reduction
The Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch working in the Dyson Perrins Laboratory in the University of Oxford, building on earlier work by Wooster and Godfrey in...
, the secondary reaction is proton abstraction from an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. This reaction type is also called a dissolving metal reduction. Alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
reduction to an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
in the liquid ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
/sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
system follows the same theme. The first radical anion intermediate abstracts a proton from ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
to the free radical. A second one-electron transfer leads to the anion, which also abstracts a proton to the neutral alkene.
In the Wurtz reaction
Wurtz reaction
The Wurtz reaction, named after Charles-Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium to form a new carbon-carbon bond:...
, two radical intermediates dimerize in a coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
. Likewise, acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
is converted to pinacol
Pinacol
Pinacol is a white solid organic compound.-Preparation:It may be produced by the pinacol coupling reaction from acetone:-Reactions:As a vicinal-diol, it can rearrange to pinacolone by the pinacol rearrangement, e.g...
with a magnesium-mercury amalgam
Amalgam (chemistry)
An amalgam is a substance formed by the reaction of mercury with another metal. Almost all metals can form amalgams with mercury, notable exceptions being iron and platinum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore.The...
in a pinacol coupling reaction. Acyloin condensation
Acyloin condensation
Acyloin condensation is a reductive coupling of two carboxylic esters using metallic sodium to yield an α-hydroxyketone, also known as an acyloin....
couples two carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s to a α-hydroxyketone. Reactions of this type are also called reductive couplings. In the Clemmensen reduction
Clemmensen reduction
Clemmensen reduction is a chemical reaction described as a reduction of ketones to alkanes using zinc amalgam and hydrochloric acid. This reaction is named after Erik Christian Clemmensen, a Danish chemist....
of ketones to alkanes with zinc-mercury amalgam, the intermediate is an organozinc carbenoid.
Electron rich organic molecules like tetrakis(dimethylamino)ethylene (TDAE) are effective reducing agents capable of generating the anion from alkyl halides such as 5-chloromethyl-6-nitrobenzo[1,3]dioxole:
The one-electron reduction potential of a molecule can be used to obtain an electron affinity. For example: The one-electron reduction potential of molecular oxygen gives a value of 1.07(1) eV.