.gif)
Steady state (chemistry)
Encyclopedia
In chemistry
, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system (compare mass balance
). One of the most simple examples of such a system is the case of a bathtub with the tap open but without the bottom plug: after a certain time the water flows in and out at the same rate, so the water level (the state variable being Volume) stabilizes and the system is at steady state.
The steady state concept is different from chemical equilibrium
. Although both may create a situation where a concentration
does not change, in a system at chemical equilibrium, the net reaction
rate is zero (products transform into reactants at the same rate as reactants transform into products), while no such limitation exists in the steady state concept. Indeed, there does not have to be a reaction at all for a steady state to develop.
The term steady state is also used to describe a situation where some, but not all, of the state variables of a system are constant. For such a steady state to develop, the system does not have to be a flow system. Therefore such a steady state can develop in a closed system where a series of chemical reactions take place. Literature in chemical kinetics
usually refers to this case, calling it steady state approximation.
In simple systems the steady state is approached by state variables gradually decreasing or increasing until they reach their steady state value. In more complex systems state variable might fluctuate around the theoretical steady state either forever (a limit-cycle
) or gradually coming closer and closer. It theoretically takes an infinite time to reach steady state, just as it takes an infinite time to reach chemical equilibrium.
Both concepts are, however, frequently used approximation
s because of the substantial mathematical simplifications these concepts offer. Whether or not these concepts can be used depends on the error the underlying assumptions introduce. So, even though a steady state, from a theoretical point of view, requires constant drivers (e.g. constant inflow rate and constant concentrations in the inflow), the error introduced by assuming steady state for a system with non-constant drivers may be negligible if the steady state is approached fast enough (relatively speaking).
in a reaction mechanism
equal to zero.
It is important to note that steady state approximation does not assume the reaction intermediate
concentration to be constant (and therefore its time derivative being zero), it assumes that the variation in the concentration of the intermediate is almost zero: the concentration of the intermediate is very low, so even a big relative variation in its concentration is small, if considered quantitatively.
Its use facilitates the resolution of the differential equation
s that arise from rate equation
s, which lack an analytical solution
for most mechanisms beyond the most simple ones. The steady state approximation is applied, for example in Michaelis-Menten kinetics
.
As an example, the steady state approximation will be applied to two consecutive, irreversible, homogeneous first order reactions in a closed system. (For heterogeneous reactions, see reactions on surfaces
.) This model corresponds, for example, to a series of nuclear decompositions
like .
.
If the rate constants for the following reaction are and
and  ;
;  , combining the rate equation
, combining the rate equation
s with a mass balance
for the system yields:

For reactant B:
For reactant C:



 therefore
therefore  so
so  .
.
 The analytical and approximated solutions should now be compared in order to decide when it is valid to use the steady state approximation. The analytical solution transforms into the approximate one when
The analytical and approximated solutions should now be compared in order to decide when it is valid to use the steady state approximation. The analytical solution transforms into the approximate one when  , because then
, because then  and
and  . Therefore it is valid to apply the steady state approximation only if the second reaction is much faster than the first one (k2/k1 > 10 is a right criterion), because that means that the intermediate
. Therefore it is valid to apply the steady state approximation only if the second reaction is much faster than the first one (k2/k1 > 10 is a right criterion), because that means that the intermediate
forms slowly and reacts readily so its concentration stays low.
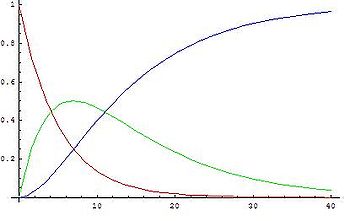
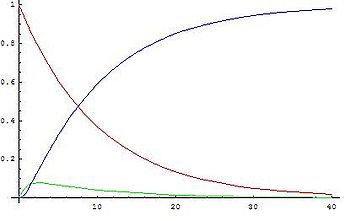
The graphs show concentrations of A (red), B (green) and C (blue) in two cases, calculated from the analytical solution:
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system (compare mass balance
Mass balance
A mass balance is an application of conservation of mass to the analysis of physical systems. By accounting for material entering and leaving a system, mass flows can be identified which might have been unknown, or difficult to measure without this technique...
). One of the most simple examples of such a system is the case of a bathtub with the tap open but without the bottom plug: after a certain time the water flows in and out at the same rate, so the water level (the state variable being Volume) stabilizes and the system is at steady state.
The steady state concept is different from chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
. Although both may create a situation where a concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
does not change, in a system at chemical equilibrium, the net reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
rate is zero (products transform into reactants at the same rate as reactants transform into products), while no such limitation exists in the steady state concept. Indeed, there does not have to be a reaction at all for a steady state to develop.
The term steady state is also used to describe a situation where some, but not all, of the state variables of a system are constant. For such a steady state to develop, the system does not have to be a flow system. Therefore such a steady state can develop in a closed system where a series of chemical reactions take place. Literature in chemical kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
usually refers to this case, calling it steady state approximation.
In simple systems the steady state is approached by state variables gradually decreasing or increasing until they reach their steady state value. In more complex systems state variable might fluctuate around the theoretical steady state either forever (a limit-cycle
Limit-cycle
In mathematics, in the area of dynamical systems, a limit-cycle on a plane or a two-dimensional manifold is a closed trajectory in phase space having the property that at least one other trajectory spirals into it either as time approaches infinity or as time approaches negative infinity. Such...
) or gradually coming closer and closer. It theoretically takes an infinite time to reach steady state, just as it takes an infinite time to reach chemical equilibrium.
Both concepts are, however, frequently used approximation
Approximation
An approximation is a representation of something that is not exact, but still close enough to be useful. Although approximation is most often applied to numbers, it is also frequently applied to such things as mathematical functions, shapes, and physical laws.Approximations may be used because...
s because of the substantial mathematical simplifications these concepts offer. Whether or not these concepts can be used depends on the error the underlying assumptions introduce. So, even though a steady state, from a theoretical point of view, requires constant drivers (e.g. constant inflow rate and constant concentrations in the inflow), the error introduced by assuming steady state for a system with non-constant drivers may be negligible if the steady state is approached fast enough (relatively speaking).
Steady state approximation in chemical kinetics
The steady state approximation, occasionally called the stationary-state approximation, involves setting the rate of change of a reaction intermediateReaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
in a reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
equal to zero.
It is important to note that steady state approximation does not assume the reaction intermediate
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
concentration to be constant (and therefore its time derivative being zero), it assumes that the variation in the concentration of the intermediate is almost zero: the concentration of the intermediate is very low, so even a big relative variation in its concentration is small, if considered quantitatively.
Its use facilitates the resolution of the differential equation
Differential equation
A differential equation is a mathematical equation for an unknown function of one or several variables that relates the values of the function itself and its derivatives of various orders...
s that arise from rate equation
Rate equation
The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with concentrations or pressures of reactants and constant parameters . To determine the rate equation for a particular system one combines the reaction rate with a mass balance for the system...
s, which lack an analytical solution
Closed-form expression
In mathematics, an expression is said to be a closed-form expression if it can be expressed analytically in terms of a bounded number of certain "well-known" functions...
for most mechanisms beyond the most simple ones. The steady state approximation is applied, for example in Michaelis-Menten kinetics
Michaelis-Menten kinetics
In biochemistry, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating...
.
As an example, the steady state approximation will be applied to two consecutive, irreversible, homogeneous first order reactions in a closed system. (For heterogeneous reactions, see reactions on surfaces
Reactions on surfaces
By reactions on surfaces it is understood reactions in which at least one of the steps of the reaction mechanism is the adsorption of one or more reactants...
.) This model corresponds, for example, to a series of nuclear decompositions
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
like
 .
.If the rate constants for the following reaction are
 and
and  ;
;  , combining the rate equation
, combining the rate equationRate equation
The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with concentrations or pressures of reactants and constant parameters . To determine the rate equation for a particular system one combines the reaction rate with a mass balance for the system...
s with a mass balance
Mass balance
A mass balance is an application of conservation of mass to the analysis of physical systems. By accounting for material entering and leaving a system, mass flows can be identified which might have been unknown, or difficult to measure without this technique...
for the system yields:
Reaction rates
For reactant A:
For reactant B:

For reactant C:

Analytical Solutions
The analytical solutions for these equations (supposing that initial concentrations of every substance except for A are zero) are :


Steady State
If the steady state approximation is applied then, the derivative of the concentration of the intermediate is set to zero. therefore
therefore  so
so  .
.Validity


 , because then
, because then  and
and  . Therefore it is valid to apply the steady state approximation only if the second reaction is much faster than the first one (k2/k1 > 10 is a right criterion), because that means that the intermediate
. Therefore it is valid to apply the steady state approximation only if the second reaction is much faster than the first one (k2/k1 > 10 is a right criterion), because that means that the intermediateReactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
forms slowly and reacts readily so its concentration stays low.
The graphs show concentrations of A (red), B (green) and C (blue) in two cases, calculated from the analytical solution:
- When the first reaction is faster it is not valid to assume that the variation of [B] is very small, because [B] is neither low or close to constant: first A transforms into B rapidly and B accumulates because it disappears slowly. As the concentration of A decreases its rate of transformation decreases, at the same time the rate of reaction of B into C increases as more B is formed, so a maximum is reached when
 . From then on the concentration of B decreases.
. From then on the concentration of B decreases.
- When the second reaction is faster, after a short induction periodInduction periodAn induction period in chemical kinetics is an initial slow stage of a chemical reaction; after the induction period, the reaction accelerates. Ignoring induction periods can lead to runaway reactions....
, concentration of B remains low (and more or less constant) because its rate of formation and disappearance are almost equal and the steady state approximation can be used.
The equilibrium approximation can be used sometimes in chemical kinetics to yield similar results as the steady state approximation (Michaelis-Menten kineticsMichaelis-Menten kineticsIn biochemistry, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating...
can be derived assuming equilibrium instead of steady state): it consists in assuming that the intermediate is at chemical equilibrium. Normally the requirements for applying the steady state approximation are laxer: the concentration of the intermediate is only needed to be low and more or less constant (as seen, this has to do only with the rates at which it appears and disappears) but it is not needed to be at equilibrium, which is usually difficult to prove and involves heavier assumptions.
External links
- When the second reaction is faster, after a short induction period

