Benzyl
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, benzyl is the term used to describe the substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
ring attached to a CH2 group.
Nomenclature
The term benzyl refers most commonly to benzyl compounds, such as benzyl chlorideBenzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.-Preparation:...
or benzyl benzoate
Benzyl benzoate
Benzyl benzoate is the ester of benzyl alcohol and benzoic acid, with the formula C6H5CH2O2CC6H5. This easily prepared compound has a variety of uses.-Synthesis:This colorless liquid is formally the condensation product of benzoic acid and benzyl alcohol...
. Benzyl also refers to a free radical with the formula C6H5CH2. The benzyl carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
has the formula C6H5CH2+. The benzyl carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
has the formula C6H5CH2-. None of these species have significant existence under normal conditions, but they are useful references for discussion of mechanisms.
Sometimes, benzyl and phenyl are confused, but their formulas and behavior is very different. The term benzylic refers to the position on a carbon skeleton next to a phenyl or other aromatic ring.
Abbreviations
The abbreviation "Bn" is frequently used to denote benzyl groups in nomenclature and structural depictions of chemical compounds. For example, benzyl alcoholBenzyl alcohol
Benzyl alcohol is an organic compound with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn", thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor...
can be represented as BnOH. This abbreviation is not to be confused with "Bz", which is the abbreviation for the benzoyl
Benzoyl
In organic chemistry, benzoyl is the acyl of benzoic acid, with structure C6H5CO-. It should not be confused with benzyl, which is the radical or ion formed from the removal of one of the methyl hydrogens of toluene...
group C6H5C(O)-.
Reactivity of benzylic centers
Benzylic positions are endowed with special reactivity, as in oxidation, free radical halogenationFree radical halogenation
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of heat or UV light. The reaction is used for the industrial synthesis of chloroform , dichloromethane , and hexachlorobutadiene...
, or hydrogenolysis
Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
. As a practical example, in the presence of suitable catalysts, p-xylene
Xylene
Xylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached...
oxidizes exclusively at the benzylic positions to give terephthalic acid
Terephthalic acid
Terephthalic acid is the organic compound with formula C6H42. This colourless solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several billion kilograms are produced annually...
:
- CH3C6H4CH3 + 3 O2 → HO2CC6H4CO2H + 2 H2O
Millions of tonnes of terephthalic acid are produced annually by this method.
The enhanced reactivity of benzylic positions is attributed to the low bond dissociation energy
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
for benzylic C-H bonds. Specifically, the bond C6H5CH2-H is about 10-15% weaker than other kinds of C-H bonds. The neighboring aromatic ring stabilizes benzyl radicals.
Benzyl protective groups
Benzyl groups are frequently used in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
as protective group for alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s and carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s.
Two common methods for benzyl ether protection:
- reaction of alcohol with benzyl bromide and a strong base such as sodium hydrideSodium hydrideSodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
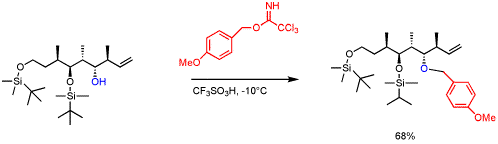
as in a Williamson ether synthesisWilliamson ether synthesisThe Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and an alcohol. This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction... - reaction of alcohol with an imidate such as benzyl trichloroacetimidate (C6H5CH2OC(CCl3)=NH) promoted by trifluoromethanesulfonic acidTrifluoromethanesulfonic acidTrifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids. Triflic acid is mainly used in research as a catalyst for esterification.-Properties:Triflic acid is a hygroscopic, colorless...
. An example forming a p-methoxybenzyl (PMB) ether in total synthesis:
The benzyl group can be removed by hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
. PMB ethers can be cleaved by magnesium bromide
Magnesium bromide
Magnesium bromide is a chemical compound of magnesium and bromine that is white and deliquescent. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. It is water soluble and somewhat soluble in alcohol...
–dimethyl sulfide
Dimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
, CAN or DDQ
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone is the chemical reagent with formula C8Cl2N2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols and steroid ketones in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.-Preparation:Synthesis of DDQ...
.
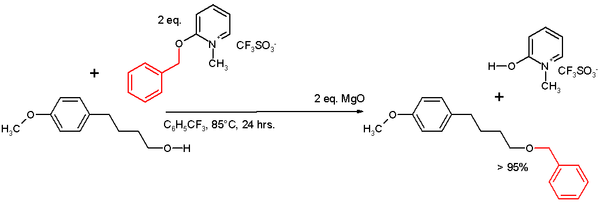
One study employs a benzyloxy pyridinium salt as a benzyl transfer reagent for alcohols:
Trifluorotoluene
Trifluorotoluene
Trifluorotoluene is an organic compound with the formula of C6H5CF3. This colorless fluorocarbon is used as a specialty solvent in organic synthesis and an intermediate in the production of pesticides and pharmaceuticals.-Synthesis:...
was used as the solvent in the presence of magnesium oxide
Magnesium oxide
Magnesium oxide , or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium . It has an empirical formula of and consists of a lattice of Mg2+ ions and O2– ions held together by ionic bonds...
as an acid scavenger
Scavenger (chemistry)
A scavenger in chemistry is a chemical substance added to a mixture in order to remove or inactivate impurities or unwanted reaction products. Their use is wide-ranged:...
. The reaction type for this conversion is believed to be SN1 based on the detection of trace amounts of Friedel-Crafts reaction
Friedel-Crafts reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877. There are two main types of Friedel–Crafts reactions: alkylation reactions and acylation reactions. This reaction type is a form of electrophilic aromatic substitution...
side-products with toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
as a solvent.