Nucleophilic aromatic substitution
Encyclopedia
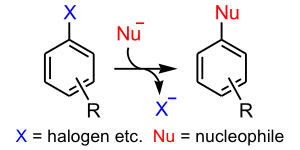
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
in which the nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
displaces a good leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
, such as a halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
, on an aromatic ring. There are 6 nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
mechanisms encountered with aromatic systems:
- the SNAr (addition-elimination) mechanism

- the aromatic SN1 mechanism encountered with diazonium salts
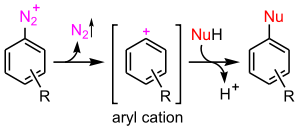
- the benzyne mechanism
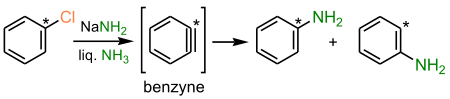
- the free radical SRN1 mechanism
- the ANRORC mechanismANRORC mechanismThe ANRORC mechanism in organic chemistry describes a special type of substitution reaction. ANRORC stands for Addition of the Nucleophile, Ring Opening, and Ring Closure in nucleophilic attack on ring systems and it helps to explain product formation and distribution in some nucleophilic...
- Vicarious nucleophilic substitutionVicarious nucleophilic substitutionVicarious nucleophilic substitution in organic chemistry is a special type of nucleophilic aromatic substitution in which a nucleophile replaces hydrogen and not an aromatic substituent like a halogen that is ordinarily encountered in this reaction type...
.
The most important of these is the SNAr mechanism, where electron withdrawing groups activate the ring towards nucleophilic attack, for example if there are nitro functional groups positioned ortho or para to the halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
leaving group.
SNAr reaction mechanism
Aryl halidesHalogenoarene
In organic chemistry, a halogenoarene, haloarene, or aryl halide, is an organic compound in which a halogen atom is bonded to a carbon atom which is part of an aromatic ring. The haloarene are studied separately from haloalkanes because they exhibit many differences in methods of preparation and...
cannot undergo SN2 reaction
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
.The C–Br bond is in the plane of the ring as the carbon atom is trigonal. To attack from the back, the nucleophile would have to appear inside the benzene ring and invert the carbon atom in an absurd way. This reaction is not possible.
SN1 reaction
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
is possible but very unfavourable. It would involve the unaided loss of the leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
and the formation of an aryl cation.
The following is the reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of a nucleophilic aromatic substitution of 2,4-dinitrochlorobenzene in a basic
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
aqueous solution.
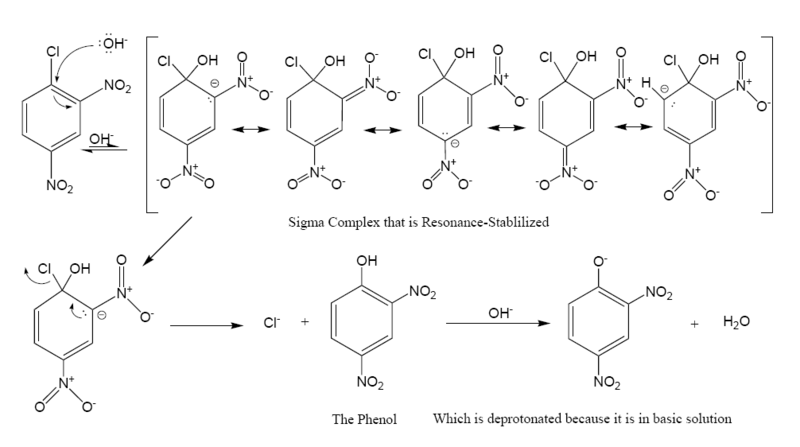
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
group is an activator toward nucleophilic substitution, and an ortho/para director, it allows the benzene carbon to which it is bonded to have a negative charge. In the Meisenheimer complex
Meisenheimer complex
A Meisenheimer complex or Jackson-Meisenheimer complex in organic chemistry is a 1:1 reaction adduct between an arene carrying electron withdrawing groups and nucleophile...
, the nonbonded electrons of the carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
become bonded to the aromatic pi system which allows the ipso carbon to temporarily bond with the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group (-OH). In order to return to a lower energy state, either the hydroxyl group leaves, or the chloride leaves. In solution both processes happen. A small percentage of the intermediate loses the chloride to become the product (2,4-dinitrophenol), while the rest return to the reactant. Since 2,4-dinitrophenol is in a lower energy state it will not return to form the reactant, so after some time has passed, the reaction reaches chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
.
The formation of the resonance-stabilized Meisenheimer complex
Meisenheimer complex
A Meisenheimer complex or Jackson-Meisenheimer complex in organic chemistry is a 1:1 reaction adduct between an arene carrying electron withdrawing groups and nucleophile...
is slow because it is in a higher energy state than the aromatic reactant. The loss of the chloride is fast, because the ring becomes aromatic again.
Nucleophilic aromatic substitution reactions
Some typical substitution reactions on arenes are listed below.- In the Bamberger rearrangementBamberger rearrangementThe Bamberger rearrangement is the chemical reaction of N-phenylhydroxylamines with strong aqueous acid, which will rearrange to give 4-aminophenols...
N-phenylhydroxylamines rearrange to 4-aminophenols. The nucleophile is water. - In the Sandmeyer reactionSandmeyer reactionThe Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts. It is named after the Swiss chemist Traugott Sandmeyer....
and the Gattermann reactionGattermann reactionThe Gattermann rection, named for the German chemist Ludwig Gattermann, in organic chemistry refers to a reaction of hydrocyanic acid with an aromatic compound, in this case benzene, under catalysis with Friedel-Crafts catalyst .Catalyst used is Copper Powder in HX in the case of reaction of...
diazonium salts react with halides. - The Smiles rearrangementSmiles rearrangementThe Smiles rearrangement is an organic reaction and a rearrangement reaction. It is an intramolecular nucleophilic aromatic substitution of the type:...
is the intramolecular version of this reaction type.
Nucleophilic aromatic substitution is not limited to arenes, however; the reaction takes place even more readily with heteroarenes. Pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
s are especially reactive when substituted in the aromatic ortho position or aromatic para position because then the negative charge is effectively delocalized at the nitrogen position. One classic reaction is the Chichibabin reaction
Chichibabin reaction
The Chichibabin reaction -chē-bā-bēn) is a method for producing 2-aminopyridine derivatives by the reaction of pyridine with sodium amide. It was reported by Aleksei Chichibabin in 1914. The following is the overall form of the general reaction:...
(Aleksei Chichibabin
Aleksei Chichibabin
For the poet, see Boris Chichibabin.Alekséy Yevgényevich Chichibábin was a Soviet/Russian organic chemist. His name is also written Alexei Yevgenievich Chichibabin and Alexei Euguenievich Tchitchibabine.- Life :...
, 1914) in which pyridine is reacted with an alkali-metal amide such as sodium amide
Sodium amide
Sodium amide, commonly called sodamide, is the chemical compound with the formula NaNH2. This solid, which is dangerously reactive toward water, is white when pure, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process...
to form 2-aminopyridine.
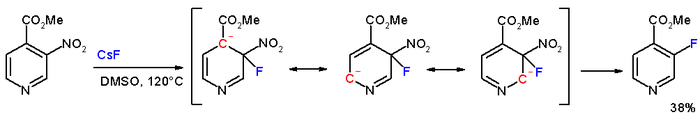
In the compound methyl 3-nitropyridine-4-carboxylate, the meta nitro group is actually displaced by fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
with caesium fluoride
Caesium fluoride
Caesium fluoride , is an inorganic compound usually encountered as a hygroscopic white solid. It is more soluble and more readily dissociated than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed it is easy to dry by heating at 100 °C for...
in DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
at 120°C.
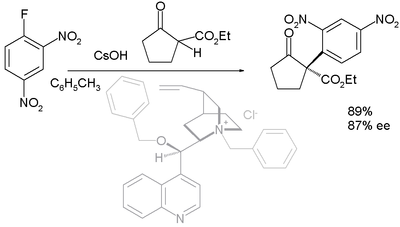
Asymmetric nucleophilic aromatic substitution
With prochiralProchiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step.If two identical substituents are attached to a sp3-hybridized atom, the descriptors pro-R and pro-S are used to distinguish between the two...
carbon nucleophiles such as 1,3-dicarbonyl compounds the reaction has been demonstrated as an asymmetric synthesis in asymmetric nucleophilic aromatic substitution. First reported in 2005, the organocatalyst (in a dual role with that of a phase transfer catalyst
Phase transfer catalyst
In chemistry, a phase transfer catalyst or PTC is a catalyst that facilitates the migration of a reactant from one phase into another phase where reaction occurs. Phase transfer catalysis is a special form of heterogeneous catalysis. Ionic reactants are often soluble in an aqueous phase but...
) is derived from cinchonidine
Cinchonidine
Cinchonidine is an alkaloid used in asymmetric synthesis in organic chemistry. It is a stereoisomer and pseudo-enantiomer of cinchonine....
(benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
ated at N and benzoylated at 9-OH):
See also
- Electrophilic aromatic substitutionElectrophilic aromatic substitutionElectrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
- NucleophileNucleophileA nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
- Substitution reactionSubstitution reactionIn a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
- SN1 reactionSN1 reactionThe SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
- SN2 reactionSN2 reactionThe SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
- SNi reaction
- Nucleophilic aliphatic substitution
- Nucleophilic acyl substitutionNucleophilic acyl substitutionNucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...