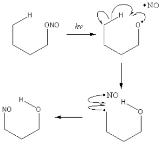
Barton reaction
Encyclopedia
The Barton Reaction involves the photolysis of a nitrite
to form a δ-nitroso
alcohol
. It is named for the British chemist Sir Derek Harold Richard Barton
. The mechanism is believed to involve a homolytic RO–NO cleavage, followed by δ-hydrogen abstraction and free radical recombination.
A related reaction is the Hofmann-Löffler reaction
involving haloamines.
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
to form a δ-nitroso
Nitroso
Nitroso refers to a functional group in organic chemistry which has the general formula RNO. Nitroso compounds are a class of organic compounds containing the nitroso functional group, R−N=O....
alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. It is named for the British chemist Sir Derek Harold Richard Barton
Derek Harold Richard Barton
Sir Derek Harold Richard Barton FRS was a British organic chemist and Nobel Prize laureate.-Biography:Barton was born to William Thomas and Maude Henrietta Barton. He attended Tonbridge School and in 1938 he entered Imperial College London, where he graduated in 1940 and obtained his Ph.D. degree...
. The mechanism is believed to involve a homolytic RO–NO cleavage, followed by δ-hydrogen abstraction and free radical recombination.
A related reaction is the Hofmann-Löffler reaction
Hofmann-Löffler reaction
The Hofmann-Löffler reaction is an organic reaction in which a cyclic amine 2 is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid...
involving haloamines.

