
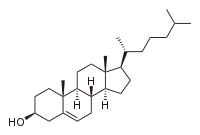
Cholesterol total synthesis
Encyclopedia

Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
describes the total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of the complex biomolecule
Biomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
and is considered a great scientific achievement. . The research group of Robert Robinson with John Cornforth
John Cornforth
Sir John Warcup 'Kappa' Cornforth, AC, CBE, FRS , is an Australian scientist who won the Nobel Prize in Chemistry in 1975 for his work on the stereochemistry of enzyme-catalyzed reactions....
(Oxford University) published their synthesis in 1951 and that of of Robert Burns Woodward
Robert Burns Woodward
Robert Burns Woodward was an American organic chemist, considered by many to be the preeminent organic chemist of the twentieth century...
with Franz Sondheimer
Franz Sondheimer
-Early life:Sondheimer was born in Stuttgart, Germany in 1926 and, following the rise of the Nazis, fled to the United Kingdom in 1937.-Education:He was a pupil at Highgate School and subsequently studied chemistry, receiving his degree from Imperial College London.-Career:From 1949 to 1952,...
(Harvard University
Harvard University
Harvard University is a private Ivy League university located in Cambridge, Massachusetts, United States, established in 1636 by the Massachusetts legislature. Harvard is the oldest institution of higher learning in the United States and the first corporation chartered in the country...
) in 1952 . Both groups competed for the first publication since 1950 with Robinson having started in 1932 and Woodward in 1949. According to historian Greg Mulheirn the Robinson effort was hampered by his micromanagement style of leadership and the Woodward effort was greatly facilitated by his good relationships with chemical industry. Around 1949 steroids like cortisone
Cortisone
Cortisone is a steroid hormone. It is one of the main hormones released by the adrenal gland in response to stress. In chemical structure, it is a corticosteroid closely related to corticosterone. It is used to treat a variety of ailments and can be administered intravenously, orally,...
were produced from natural resources but expensive. Chemical companies Merck & Co.
Merck & Co.
Merck & Co., Inc. , also known as Merck Sharp & Dohme or MSD outside the United States and Canada, is one of the largest pharmaceutical companies in the world. The Merck headquarters is located in Whitehouse Station, New Jersey, an unincorporated area in Readington Township...
and Monsanto
Monsanto
The Monsanto Company is a US-based multinational agricultural biotechnology corporation. It is the world's leading producer of the herbicide glyphosate, marketed in the "Roundup" brand of herbicides, and in other brands...
saw commercial opportunities for synthesis steroids and not only funded Woodward but also provided him with large quantities of certain chemical intermediates from pilot plants. Hard work also helped the Woodward effort: one of the intermediate compounds was named Christmasterone as it was synthesized on Christmas Day 1950 by Sondheimer.
Other cholesterol schemes have also been developed: racemic cholesterol was synthesized in 1966 by W.S. Johnson , the cholesterol enantomer was reported in 1996 by Rychnovsky and Mickus and in 2002 by Jiang & Covey
The molecule
Cholesterol is a tetracyclicTetracyclic
Tetracyclic may refer to* any of a number of cyclic compounds containing four rings. They have various pharmaceutical uses; see Tetracyclic drug group.* cyclic flowers constructed of four whorls....
alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
and a type of sterol
Sterol
Sterols, also known as steroid alcohols, are a subgroup of the steroids and an important class of organic molecules. They occur naturally in plants, animals, and fungi, with the most familiar type of animal sterol being cholesterol...
. Added to the sterol frame with the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
group at position 3 are 2 methyl groups at carbon positions 10 and 13 and an 2-isooctyl group at position 17. The molecule is unsaturated at position 5,6 with an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
group. The total number of stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s is 8. The unnatural cholesterol molecule that has also been synthesized is called ent-cholesterol.
Robinson synthesis
The Robinson synthesis is an example of a so-called relay synthesis. As many of the chemical intermediates (all steroids) were already known and available from natural resources all that was needed for a formal synthesis was proof that these intermediates could be linked to each other via chemical synthesis. Starting point for the Robinson synthesis was 1,6-dihydroxynapthalene 1 that was converted in about 20 steps into the then already known androsteroneAndrosterone
Androsterone is a steroid hormone with weak androgenic activity. It is made in the liver from the metabolism of testosterone. Its beta-isomer is Epiandrosterone.-History:...
4. Ruzicka had already demonstrated in 1938 that androsterone could be converted into androstenedione
Androstenedione
Androstenedione is a 19-carbon steroid hormone produced in the adrenal glands and the gonads as an intermediate step in the biochemical pathway that produces the androgen testosterone and the estrogens estrone and estradiol.-Synthesis:Androstenedione is the common precursor of male and female sex...
5 and Robinson demonstrated its conversion to dehydroepiandrosterone
Dehydroepiandrosterone
5-Dehydroepiandrosterone is a 19-carbon endogenous steroid hormone. It is the major secretory steroidal product of the adrenal glands and is also produced by the gonads and the brain. DHEA is the most abundant circulating steroid in humans....
6 (note the epimerized
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
hydroxyl group) also already a known compound . Conversion of 6 to pregnenolone
Pregnenolone
Pregnenolone is a steroid hormone involved in the steroidogenesis of progesterone, mineralocorticoids, glucocorticoids, androgens, and estrogens. As such it is a prohormone. Pregnenolone sulfate is a GABAA antagonist and increases neurogenesis in the hippocampus.-Chemistry:Like other steroids,...
7 and then to allopregnanolone
Allopregnanolone
Allopregnanolone is a prototypic neurosteroid present in the blood and also the brain. It is a metabolite of progesterone and potent modulator of GABAA receptors...
8 allowed the addition of the tail group as the acetate in 9 and then conversion to cholestanol 10.
The conversion of cholestanol to cholesterol was already demonstrated by oxidation of the ketone, bromination to the bromoketone
Haloketone
A haloketone in organic chemistry is a functional group consisting of a ketone group or more general a carbonyl group with a α-halogen substituent. The general structure is RR'CCR where R is an alkyl or aryl residue and X any one of the halogens...
and elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
to the enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
.
The conversion of cholestenone into cholesterol by the method of Dauben and Eastham (1950) consisted of reduction of the enol acetate (lithium aluminum hydride) and fractionation with digitonin
Digitonin
Digitonin is a glycoside obtained from Digitalis purpurea; the aglycone is digitogenin, a spirostan steroid. Used as a detergent, it effectively water-solubilizes lipids...
for the isolation of the correct isomer.
Woodward synthesis
Starting point for the Woodward synthesis was the hydroquinoneHydroquinone
Hydroquinone, also benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, having the chemical formula C6H42. Its chemical structure, shown in the table at right, has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid...
1 that was converted to cis-bicycle 2 in a Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
with butadiene. Conversion to the desired trans isomer 5 was accomplished by synthesis of the sodium enolate salt 4 (benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, sodium hydride
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
) followed by acidification. Reduction (lithium aluminum hydride) then gave diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
6, an dehydration
Dehydration
In physiology and medicine, dehydration is defined as the excessive loss of body fluid. It is literally the removal of water from an object; however, in physiological terms, it entails a deficiency of fluid within an organism...
(HCl
HCL
HCL or HCl can stand for:* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia* Hardware compatibility list...
/water) gave ketol 7, deoxygenation
Deoxygenation
Deoxygenation is a chemical reaction involving the removal of molecular oxygen from a reaction mixture or solvent, or the removal of oxygen atoms from a molecule.Classic representatives of deoxygenation are:...
of its acetate by elemental zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
gave enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
8, formylation
Formylation
Formylation is a type of posttranslational modification in which a formyl group is added to the N-terminus of a protein....
(ethyl formate
Ethyl formate
Ethyl formate is an ester formed when ethanol reacts with formic acid . It is also known as ethyl methanoate because formic acid is also known as methanoic acid. Ethyl formate has the characteristic smell of rum and is also partially responsible for the flavor of raspberries.-Exposure:Ethyl...
) gave enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
9, Michael ethyl vinyl ketone addition (potassium t-butoxide/t-butanol) gave dione 11 which on reaction with KOH
KOH
-Chemistry:*Potassium hydroxide, formula "KOH"** KOH test, a procedure in which potassium hydroxide is used to dissolve skin and reveal fungal cells under the microscope** KOH titration, a procedure for determining a lubricant's acid level or TBN-Person's name:...
in dioxane gave tricycle 12 in an aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
with elimination of the formyl group. In the next series of steps oxidation (osmium tetroxide) gave diol 13, protection (acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
/copper sulfate) gave acetonide 14, hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
(palladium-strontium carbonate) gave 15, formylation
Formylation
Formylation is a type of posttranslational modification in which a formyl group is added to the N-terminus of a protein....
(ethyl formate
Ethyl formate
Ethyl formate is an ester formed when ethanol reacts with formic acid . It is also known as ethyl methanoate because formic acid is also known as methanoic acid. Ethyl formate has the characteristic smell of rum and is also partially responsible for the flavor of raspberries.-Exposure:Ethyl...
) gave enol 16 which protected as the enamine
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
17 (methylaniline/methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
) gave via the potassium anion 18 , carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
19 by reaction with cyanoethylene using triton B as the base.
Acid 19 was converted to lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
20 (acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, sodium acetate
Sodium acetate
Sodium acetate, CH3COONa, also abbreviated NaOAc, also sodium ethanoate, is the sodium salt of acetic acid. This colourless salt has a wide range of uses.-Industrial:...
) and reaction with methylmagnesium chloride
Methylmagnesium chloride
Methylmagnesium chloride is a commercially available Grignard reagent. Like methyllithium, it is the synthetic equivalent to the methyl carbanion synthon. It is usually sold as a solution in tetrahydrofuran. The model of the molecule shows methylmagnesium chloride with the magnesium atom in the...
gave tetracyclic ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
21. Treatment with periodic acid
Periodic acid
Periodic acid, or iodic acid is an oxoacid of iodine having chemical formula HIO4 or H5IO6.In dilute aqueous solution, periodic acid exists as discrete hydronium and metaperiodate ions. When more concentrated, orthoperiodic acid, H5IO6, is formed; this dissociates into hydronium and...
(dioxane) and piperidine aceate (benzene) gave aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
24 through diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
22 (oxidation) and dialdehyde 23 (aldol condensation). Sodium dichromate
Sodium dichromate
Sodium dichromate is the chemical compound with the formula Na2Cr2O7. Usually, however, the salt is handled as its dihydrate Na2Cr2O7·2H2O. Virtually all chromium ore is processed via conversion to sodium dichromate. In this way, many millions of kilograms of sodium dichromate are produced...
oxidation gave carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
25, Diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
treatment gave methyl ester 26 and sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
the allyl alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
27. Chiral resolution
Chiral resolution
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs...
of this racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
compound with digitonin
Digitonin
Digitonin is a glycoside obtained from Digitalis purpurea; the aglycone is digitogenin, a spirostan steroid. Used as a detergent, it effectively water-solubilizes lipids...
produced chiral 28 and on Oppenauer oxidation
Oppenauer oxidation
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.The reaction is the opposite of Meerwein-Ponndorf-Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess acetone...
chiral 29. Hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
(Adams' catalyst
Adams' catalyst
Adams' catalyst, also known as platinum dioxide, is usually represented as platinum oxide hydrate, PtO2-H2O. It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available...
) gave alcohol 30, chromic acid
Chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the...
oxidation gave ketone 31, sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
reduction stereoselectively gave alcohol 32, hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
followed by acylation
Acylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
gave acetate 33, thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
treatment gave acyl chloride
Acyl chloride
In organic chemistry, an acyl chloride is an organic compound with the functional group -CO-Cl. Their formula is usually written RCOCl, where R is a side chain. They are usually considered to be reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride,...
34 and methyl cadmium the ketone 35.
In the final stages reaction of 35 with isohexylmagnesium bromide 36 gave diol 37, acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
treatment gave dehydration
Dehydration
In physiology and medicine, dehydration is defined as the excessive loss of body fluid. It is literally the removal of water from an object; however, in physiological terms, it entails a deficiency of fluid within an organism...
and then hydrogenation gave acetate 38. Hydrolysis of this ester gave cholestanol 39. The route from cholestanol to cholesterol was already known (see: Robinson synthesis).

