
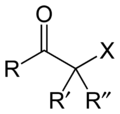
Haloketone
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
consisting of a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
group or more general a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group with a α-halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
. The general structure is RR'C(X)C(=O)R where R is an alkyl or aryl residue and X any one of the halogens. The preferred conformation
Chemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
of a haloketone is that of a cisoid
Cisoid
Cisoid may refer to:* Geometric isomerism, form of geometric isomer* Complex Sinusoid, complex sinusoidal function...
with the halogen and carbonyl sharing the same plane as the steric hindrance with the carbonyl alkyl group is generally larger.
Haloketone synthesis
- Haloketones and halo carbonyl compounds in general are synthesized by reaction of carbonylCarbonylIn organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds with halogenation agents:- HalogenHalogenThe halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s, bromine and chlorine give monosubstitution, fluorineFluorineFluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
gives polysubstitution - Tetrabutylammonium tribromideTetrabutylammonium tribromideTetrabutylammonium tribromide is a reagent used in organic chemistry as a source of bromine. Its melting point ranges from 103-104 °C....
- N-BromosuccinimideN-BromosuccinimideN-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
- Halogen
- In the Hell-Volhard-Zelinsky halogenationHell-Volhard-Zelinsky halogenationThe Hell-Volhard-Zelinsky halogenation reaction halogenates carboxylic acids at the α carbon. The reaction is named after three chemists, the German chemists Carl Magnus von Hell and Jacob Volhard and the Russian chemist Nikolay Zelinsky .- Scheme :Unlike other halogenation reactions, this...
a carboxylic acidCarboxylic acidCarboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
reacts with bromine in presence of phosphorus tribromidePhosphorus tribromidePhosphorus tribromide is a colourless liquid with the formula PBr3. It fumes in air due to hydrolysis and has a penetrating odour. It is widely used in the laboratory for the conversion of alcohols to alkyl bromides.-Preparation:...
. - In the Nierenstein reactionNierenstein reactionThe Nierenstein reaction is an organic reaction describing the conversion of an acid chloride into an haloketone with diazomethane. It is an insertion reaction in that the methylene from the diazomethane is inserted into the carbon-chlorine bond of the acid chloride.-Reaction mechanism:Like the...
an acyl chloride reacts with diazomethaneDiazomethaneDiazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
Asymmetric synthesis
Efforts are reported in asymmetric synthesis of halocarbonyls through organocatalysisOrganocatalysis
In organic chemistry, the term Organocatalysis refers to a form of catalysis, whereby the rate of a chemical reaction is increased by an organic catalyst referred to as an "organocatalyst" consisting of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds...
. In one study an acid chloride is converted into an α-halo-ester with a strong base (sodium hydride
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
), a bromine donor and an organocatalyst based on proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
and quinine
Quinine
Quinine is a natural white crystalline alkaloid having antipyretic , antimalarial, analgesic , anti-inflammatory properties and a bitter taste. It is a stereoisomer of quinidine which, unlike quinine, is an anti-arrhythmic...
:
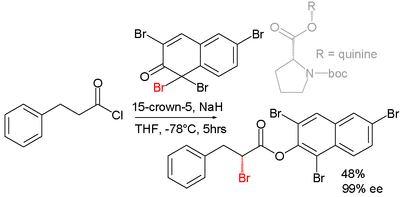
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
the base first converts the acid chloride to the ketene
Ketene
A ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
, the organocatalyst then introduces chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
through its quininoid tertiary amine, forming a ketene adduct.
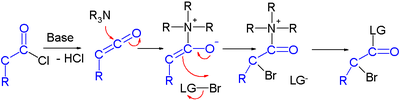
Haloketone reactions
Haloketones take part in several reaction types. In reaction with a nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
2 electrophilic sites are available and in reactions with a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
several acidic protons exist due to the presence of two electron withdrawing groups. The carbon halogen bond experiences increases polarity
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
from the inductive effect
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
of the carbonyl group making the carbon atom more electropositive.
- In nucleophilic aliphatic substitution reactions with potassium iodidePotassium iodidePotassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
in acetoneAcetoneAcetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
, 1-chloro-2-propanone reacts faster than n-propylchloride by a factor of 36000. - In the Favorskii rearrangementFavorskii rearrangementThe Favorskii rearrangement , named for the Russian chemist Alexei Yevgrafovich Favorskii, is most principally a rearrangement of cyclopropanones and α-halo ketones which leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorski rearrangement constitutes a ring...
a base abstacts first an acidic α-proton and the resulting carbanionCarbanionA carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
then displaces the halogen. - The same sequence is observed in the Bingel reactionBingel reactionThe Bingel reaction in fullerene chemistry is a fullerene cyclopropanation reaction to a methanofullerene first discovered by C. Bingel in 1993 with the bromo derivative of diethyl malonate in the presence of a base such as sodium hydride or DBU...
with fullerenes - In crossed Aldol reactionAldol reactionThe aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
s between haloketones and aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s the initial reaction product is an halohydrinHalohydrinA halohydrin or a haloalcohol is a type of organic compound or functional group in which one carbon atom has a halogen substituent, and an adjacent carbon atom has a hydroxyl substituent. They are derived from alcohols are therefore characterized by the presence of both the hydroxyl functional...
which can subsequently form a oxirane in the presence of base. - Haloketones are important in heterocyclic chemistry. An example is the use of haloketones in the Hantzsch pyrrole synthesisHantzsch pyrrole synthesisThe Hantzsch pyrrole synthesis, named for Arthur Rudolf Hantzsch, is the chemical reaction of β-ketoesters with ammonia and α-haloketones to give substituted pyrroles ....
and the Hantzsch thiazole synthesis. - Haloketones react with phosphites in the Perkow reactionPerkow reactionThe Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl phosphate and an alkyl halide....
. - The halogroup can be removed in reductive dehalogenation of halo ketonesReductive dehalogenation of halo ketonesReductive dehalogenations of halo ketones are organic reactions that result in the formation of ketones and functionalized derivatives of ketones from α-halo ketones in the presence of metallic reducing agents.-Introduction:...
- Historically, treatment of haloketones with zinc dust in the Reformatsky reaction was one of the first reliable methods for generating unstabilized enolates. This has largely been superseded by bases such as lithium diisopropylamideLithium diisopropylamideLithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
.

