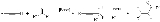
Favorskii reaction
Encyclopedia
The Favorskii reaction named for the Russian chemist Alexei Yevgrafovich Favorskii
, is a special case of nucleophilic attack on a carbonyl
group involving a terminal alkyne
with acidic protons.
 When catalyzed by acid, this reaction is called the Meyer–Schuster rearrangement.
When catalyzed by acid, this reaction is called the Meyer–Schuster rearrangement.
is formed in situ
when an alkyne
is treated with a strong bases
such as a hydroxide
or an alkoxide
. The metal acetylide then reacts with an aldehyde or ketone to form a propargyl alcohol
. When an alpha hydrogen is present (as is the case when the carbonyl is an aldehyde
), it will tautomer
ize to the corresponding enone
.
This reaction is used as a protect alkyne
s: the alkyne is either converted with acetone
to a 2-hydroxyprop-2-yl-alkyne or converted directly with the commercially available 3-methyl-1-butyne-3-ol also to a protected alkyne. The protective group can be removed by heating the compound in a solution of potassium hydroxide
in isopropanol (a retro-Favorskii reaction).
Alexei Yevgrafovich Favorskii
Alexey Yevgrafovich Favorsky, also spelled Favorskii, was a Soviet/Russian chemist.-Life:Favorsky studied chemistry at the imperial University of Saint Petersburg from 1878 to 1882. He joined Alexander Butlerov's laboratory for several years, and in 1891 became a lecturer. In 1895, Favorksy...
, is a special case of nucleophilic attack on a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group involving a terminal alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
with acidic protons.

Reaction mechanism
A metal acetylideMetal acetylide
Acetylide, ethynide, dicarbide, or percarbide is the divalent anion with formula C22− or 2−. It may be regarded as the result of removing two protons from acetylene C2H2 or H-C≡C-H, the prototypical alkyne — that behaves as a weak acid.These terms are also used for any monovalent anion...
is formed in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
when an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
is treated with a strong bases
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
such as a hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
or an alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
. The metal acetylide then reacts with an aldehyde or ketone to form a propargyl alcohol
Propargyl alcohol
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound which is a simple alcohol containing an alkyne functional group . Propargyl alcohol is a clear colorless viscous liquid that is miscible with water and most polar organic solvents. It is insoluble in most hydrocarbon solvents...
. When an alpha hydrogen is present (as is the case when the carbonyl is an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
), it will tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
ize to the corresponding enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
.
This reaction is used as a protect alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s: the alkyne is either converted with acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
to a 2-hydroxyprop-2-yl-alkyne or converted directly with the commercially available 3-methyl-1-butyne-3-ol also to a protected alkyne. The protective group can be removed by heating the compound in a solution of potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
in isopropanol (a retro-Favorskii reaction).

