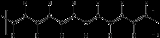
Polyacetylene
Encyclopedia
Polyacetylene is an organic polymer with the repeat unit (C2H2)n. The high electrical conductivity discovered for these polymers beginning in the 1960's accelerated interest in the use of organic compound
s in microelectronics
(organic electronics
). Polyacetylenes are also known where the H atoms are replaced with alkyl groups.
atoms with alternating single and double bond
s between them, each with one hydrogen
atom. Schematically the structure of polyacetylene is shown below.
n.png) One distinguishes trans-polyacetylene, with all double bonds in the trans configuration, from cis-polyactylene, with all double bonds in the cis configuration. Each hydrogen atom could be replaced by a functional group
One distinguishes trans-polyacetylene, with all double bonds in the trans configuration, from cis-polyactylene, with all double bonds in the cis configuration. Each hydrogen atom could be replaced by a functional group
.
: the polymerization
can be effected with anionic, cationic, and radical initiator
s. Polyacetylene is generally not prepared by polymerizing acetylene
, which is a highly flammable gas that uncontrollably oligomer
izes at high concentrations. The most common syntheses use ring opening metathesis polymerisation
("ROMP") of molecules like cyclooctatetraene
and substituted derivatives thereof.
and organic conductor).
Subsequent rediscovery of the conductive properties of oxidized doped polyacetylenes occurred in the early 1970s. A graduate student of Professor Hideki Shirakawa
accidentally polymerized acetylene with 1000 times the required amount of catalyst. The resultant polyacetylene was a silver, conductive film. Shirakawa later collaborated with physicist Alan J. Heeger
and chemist Alan G MacDiarmid, and discovered in 1976 that oxidation of this material with iodine
results in a 108-fold increase in conductivity. The conductivity of this doped material can approach the conductivity of the best available conductor, silver
. The three were awarded the Nobel Prize in Chemistry
in 2000 for their discoveries.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s in microelectronics
Microelectronics
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture of very small electronic components. Usually, but not always, this means micrometre-scale or smaller,. These devices are made from semiconductors...
(organic electronics
Organic electronics
Organic electronics, plastic electronics or polymer electronics, is a branch of electronics dealing with conductive polymers, plastics, or small molecules. It is called 'organic' electronics because the polymers and small molecules are carbon-based...
). Polyacetylenes are also known where the H atoms are replaced with alkyl groups.
Structure of polyacetylene
The polymer consists of a long chain of carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms with alternating single and double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s between them, each with one hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom. Schematically the structure of polyacetylene is shown below.
n.png)
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
.
Preparation
Acetylene polymerizes in a similar fashion to ethyleneEthylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
: the polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
can be effected with anionic, cationic, and radical initiator
Initiator
An initiator can refer to:* A person that takes an initiative in making something happen.* Modulated neutron initiator, a neutron source used in some nuclear weapons...
s. Polyacetylene is generally not prepared by polymerizing acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
, which is a highly flammable gas that uncontrollably oligomer
Oligomer
In chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
izes at high concentrations. The most common syntheses use ring opening metathesis polymerisation
Ring opening metathesis polymerisation
Ring-opening metathesis polymerization is a type of olefin metathesis chain-growth polymerization that produces industrially important products. The driving force of the reaction is relief of ring strain in cyclic olefins and a wide variety of catalysts have been discovered...
("ROMP") of molecules like cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
and substituted derivatives thereof.
Conductivity and the Nobel Prize
The 1964 monograph Organic Semiconductors, references several previous reports of high-conductivity oxidized polyacetylenes. Similarly, highly-conductive organic conductors were reported by several groups in the 1950s (see conductive polymerConductive polymer
Conductive polymers or, more precisely, intrinsically conducting polymers are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive...
and organic conductor).
Subsequent rediscovery of the conductive properties of oxidized doped polyacetylenes occurred in the early 1970s. A graduate student of Professor Hideki Shirakawa
Hideki Shirakawa
Hideki Shirakawa is a Japanese chemist and winner of the 2000 Nobel Prize in Chemistry for his discovery of conductive polymers together with physics professor Alan J. Heeger and chemistry professor Alan G...
accidentally polymerized acetylene with 1000 times the required amount of catalyst. The resultant polyacetylene was a silver, conductive film. Shirakawa later collaborated with physicist Alan J. Heeger
Alan J. Heeger
Alan Jay Heeger is an American physicist, academic and Nobel Prize laureate in chemistry.Heeger was born in Sioux City, Iowa to a Jewish family. He earned a B.S. in physics and mathematics from the University of Nebraska-Lincoln in 1957, and a Ph.D in physics from the University of California,...
and chemist Alan G MacDiarmid, and discovered in 1976 that oxidation of this material with iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
results in a 108-fold increase in conductivity. The conductivity of this doped material can approach the conductivity of the best available conductor, silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
. The three were awarded the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
in 2000 for their discoveries.

