
Corey-Fuchs reaction
Encyclopedia
The Corey–Fuchs reaction, also known as the Ramirez–Corey–Fuchs reaction, is a series of chemical reaction
s designed to transform an aldehyde
into an alkyne
. The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was originally discovered by Desai, McKelvie and Ramirez. The overall transformation of an aldehyde to an alkyne by this method is named after its discoverers, American chemists Elias James Corey
and Philip L. Fuchs.
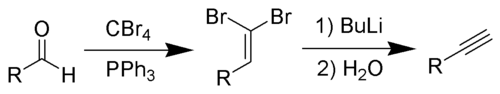 By suitable choice of base, it is often possible to stop the reaction at the 1-bromoalkyne, a useful functional group for further transformation.
By suitable choice of base, it is often possible to stop the reaction at the 1-bromoalkyne, a useful functional group for further transformation.
, where the phosphorus ylide is formed from dibromocarbene. This carbene is generated in situ from the reaction of Triphenylphosphine
and carbon tetrabromide.
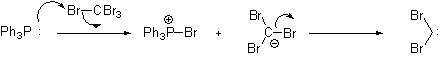 Triphenylphosphine
Triphenylphosphine
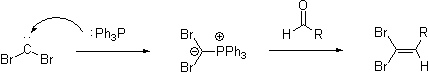
then attacks the nascent carbene
to form the reactive ylide
. This ylide undergoes a Wittig Reaction when exposed to an aldehyde.
 Deprotonation of the weakly acidic olefinic proton with butyllithium gives rise to a lithio-olefinic species which can undergo a beta-elimination to produce the bromoalkyne. Further treatment with butyllithium allows for a lithium–halogen exchange and the intermediate can be quenched with an electrophile, such as water or an alkyl halide, transforming the bromoalkyne to the terminal acetylene, or the internal alkyne, respectively.
Deprotonation of the weakly acidic olefinic proton with butyllithium gives rise to a lithio-olefinic species which can undergo a beta-elimination to produce the bromoalkyne. Further treatment with butyllithium allows for a lithium–halogen exchange and the intermediate can be quenched with an electrophile, such as water or an alkyl halide, transforming the bromoalkyne to the terminal acetylene, or the internal alkyne, respectively.
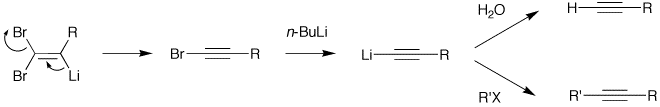
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s designed to transform an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
into an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
. The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was originally discovered by Desai, McKelvie and Ramirez. The overall transformation of an aldehyde to an alkyne by this method is named after its discoverers, American chemists Elias James Corey
Elias James Corey
Elias James Corey is an American organic chemist. In 1990 he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis...
and Philip L. Fuchs.

Reaction mechanism
The Corey–Fuchs reaction is based on a special case of the Wittig ReactionWittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
, where the phosphorus ylide is formed from dibromocarbene. This carbene is generated in situ from the reaction of Triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
and carbon tetrabromide.
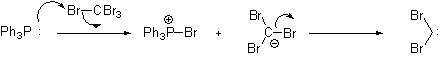
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
then attacks the nascent carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
to form the reactive ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
. This ylide undergoes a Wittig Reaction when exposed to an aldehyde.

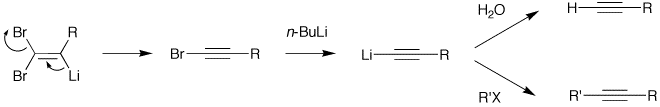
See also
- Fritsch-Buttenberg-Wiechell rearrangementFritsch-Buttenberg-Wiechell rearrangementThe Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch, Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction whereby a 1,1-diaryl-2-bromo-alkene rearranges to a 1,2-diaryl-alkyne by reaction with a strong base such as an alkoxide.This rearrangement...
- Seyferth-Gilbert homologationSeyferth-Gilbert homologationThe Seyferth–Gilbert homologation is a the chemical reaction of an aryl ketone 1 with dimethyl phosphonate 2 and potassium tert-butoxide to give substituted alkynes 3...
- Wittig reactionWittig reactionThe Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....

