
Vinyl halide
Encyclopedia
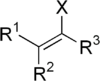
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, a vinyl halide is any alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
with at least one halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
bonded directly on one of the unsaturated carbons. Vinyl chloride
Vinyl chloride
Vinyl chloride is the organochloride with the formula H2C:CHCl. It is also called vinyl chloride monomer, VCM or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride . At ambient pressure and temperature, vinyl chloride...
is one such substance.
Vinyl halides are very useful synthetic intermediates due to the vast number of reactions that make use of them. These include conversion to vinyl Grignard reagents
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
, elimination to give the corresponding alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
, and most importantly their use in cross-coupling reactions
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
(e.g. Suzuki-Miyaura coupling
Suzuki reaction
The Suzuki reaction is the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls, and has been extended to incorporate alkyl bromides...
, Stille coupling
Stille reaction
The Stille reaction is a chemical reaction coupling an organotin compound with an sp2-hybridized organic halide catalyzed by palladium. The reaction is widely used in organic synthesis....
, Heck coupling
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
, etc.).
As a result, there is a large number of reactions to form vinyl halides, which includes the reaction of vinyl organometallic species with halogens, and the Takai
Takai olefination
Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. In the original 1986 publication the aldehyde is benzaldehyde and the organochromium species is generated from iodoform or bromoform and an excess of chromium...
and Wittig olefination
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
reactions.
these are the compounds in which halogen is bonded to a sp2 hybridised carbon atom

