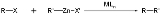
Negishi coupling
Encyclopedia
The Negishi coupling is a cross coupling reaction in organic chemistry
involving an organozinc compound
, an organic halide
and a nickel
or palladium
catalyst creating a new carbon-carbon covalent bond
:
Palladium catalysts in general have higher chemical yields and higher functional group
tolerance.
The reaction is named after Ei-ichi Negishi
who received the 2010 Nobel Prize in Chemistry
for the discovery and development of this reaction.
step of the organic halide followed by transmetalation
with the zinc compound and then reductive elimination:
Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R' resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow trans-cis isomerization first.
A common side reaction is homocoupling. In one Negishi model system the formation of homocoupling was found to be the result of a second transmetalation reaction between the diarylmetal intermediate and arylmetal halide :
from 2-bromopyridine with tetrakis(triphenylphosphine)palladium(0)
, the synthesis of a biphenyl
from o-tolylzinc chloride and o-iodotoluene and tetrakis(triphenylphosphine)palladium(0), the synthesis of 5,7-hexadecadiene from 1-decyne and (Z)-1-hexenyl iodide.
 Negishi coupling has been applied in the synthesis of hexaferrocenylbenzene:
Negishi coupling has been applied in the synthesis of hexaferrocenylbenzene:
with hexaiodidobenzene, diferrocenylzinc and tris(dibenzylideneacetone)dipalladium(0)
in tetrahydrofuran
. The yield is only 4% signifying substantial crowding around the aryl core.
In a novel modification palladium is first oxidized by the haloketone
2-chloro-2-phenylacetophenone 1 and the resulting palladium OPdCl complex then accepts both the organozinc compound
2 and the organotin compound 3 in a double transmetalation
:
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
involving an organozinc compound
Organozinc compound
Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions....
, an organic halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
and a nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
or palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
catalyst creating a new carbon-carbon covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
:
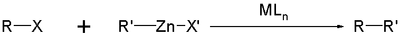
- The halide X can be chlorideChlorideThe chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
, bromineBromineBromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
or iodineIodineIodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
but also a triflateTriflateTrifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
or acetyloxy group with as the organic residue R alkenyl, arylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
, allylAllylAn allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
, alkynyl or propargylPropargylIn organic chemistry, propargyl is an alkyl functional group of 2-propynyl with the structure HC≡C−CH2−, derived from the alkyne propyne.The term propargylic refers to a saturated position on a molecular framework next to an alkynyl group...
. - The halide X' in the organozinc compound can be chlorideChlorideThe chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
, bromineBromineBromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
or iodineIodineIodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
and the organic residue R' is alkenyl, arylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
, allylAllylAn allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
or alkyl. - The metal M in the catalyst is nickelNickelNickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
or palladiumPalladiumPalladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired... - The ligandLigandIn coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
L in the catalyst can be triphenylphosphineTriphenylphosphineTriphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
, dppe, BINAPBINAPBINAP is an abbreviation for the organophosphorus compound 2,2'-bis-1,1'-binaphthyl. This chiral ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1´ positions. This C2-symmetric framework lacks stereogenic atom, but...
or chiraphosChiraphosChiraphos is a chiral diphosphine employed as a ligand in organometallic chemistry. This bidentate ligand chelates metals via the two phosphine groups. Its name is derived from its description — being both chiral and a phosphine...
- The halide X can be chloride
Palladium catalysts in general have higher chemical yields and higher functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
tolerance.
The reaction is named after Ei-ichi Negishi
Ei-ichi Negishi
is a Japanese chemist who has spent most of his career at Purdue University, United States. He is best known for his discovery of the Negishi coupling. He was awarded the 2010 Nobel Prize in Chemistry "for palladium catalyzed cross couplings in organic synthesis" jointly with Richard F. Heck and...
who received the 2010 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
for the discovery and development of this reaction.
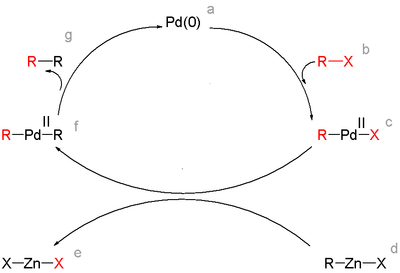
Reaction mechanism
The active catalyst in this reaction is zerovalent (M0) and the reaction in general proceeds through an oxidative additionOxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
step of the organic halide followed by transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
with the zinc compound and then reductive elimination:
Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R' resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow trans-cis isomerization first.
A common side reaction is homocoupling. In one Negishi model system the formation of homocoupling was found to be the result of a second transmetalation reaction between the diarylmetal intermediate and arylmetal halide :
- Ar–Pd–Ar' + Ar'–Zn–X → Ar'–Pd–Ar' + Ar–Zn–X
- Ar'–Pd–Ar' → Ar'–Ar' + Pd(0) (homocoupling)
- Ar–Zn–X + H2O → Ar–H + HO–Zn–X (reaction accompanied by dehalogenation)
Scope
The Negishi coupling has been applied in the synthesis of a 2,2'-bipyridine2,2'-Bipyridine
2,2'-Bipyridine is a organic compound with the formula . This colorless solid, commonly abbreviated bipy or bpy , is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals...
from 2-bromopyridine with tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0)
Tetrakispalladium is the chemical compound Pd[P3]4, often abbreviated Pd4, or even PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.-Structure and properties:...
, the synthesis of a biphenyl
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
from o-tolylzinc chloride and o-iodotoluene and tetrakis(triphenylphosphine)palladium(0), the synthesis of 5,7-hexadecadiene from 1-decyne and (Z)-1-hexenyl iodide.

with hexaiodidobenzene, diferrocenylzinc and tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0)
Trisdipalladium or Pd23 is an organometallic complex based on palladium and dibenzylideneacetone used in organic chemistry. It was discovered in 1970.-Preparation and structure:...
in tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
. The yield is only 4% signifying substantial crowding around the aryl core.
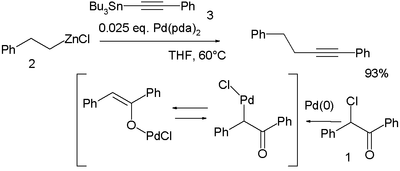
In a novel modification palladium is first oxidized by the haloketone
Haloketone
A haloketone in organic chemistry is a functional group consisting of a ketone group or more general a carbonyl group with a α-halogen substituent. The general structure is RR'CCR where R is an alkyl or aryl residue and X any one of the halogens...
2-chloro-2-phenylacetophenone 1 and the resulting palladium OPdCl complex then accepts both the organozinc compound
Organozinc compound
Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions....
2 and the organotin compound 3 in a double transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
:
External links
- The Negishi coupling at www.organic-chemistry.org: Link
- Negishi coupling - synthetic protocols from organic-reaction.com