
Stille reaction
Encyclopedia
The Stille reaction is a chemical reaction
coupling an organotin compound with an sp2-hybridized organic halide
catalyzed by palladium
. The reaction is widely used in organic synthesis
.
 X is typically a halide
X is typically a halide
, such as Cl
, Br
, I
. Additionally, X can be a pseudohalide such as a triflate
, C
F
3S
O
3-.
The Stille reaction was discovered in 1977 by John Kenneth Stille
and David Milstein
, a post-doctorate in his laboratory. Stille reactions were used in 50% of all cross-coupling reactions published in 1992. The reaction continues to be exploited industrially, especially for pharmaceuticals.
The reaction is usually performed under inert atmosphere
using dehydrated
and degassed solvent
, as oxygen causes the oxidation of the palladium catalyst and promotes homo-coupling of organic stannyl
compounds, and these side reactions lead to a decrease in the yield of the desired cross-coupling reaction.
As the organic tin compound, a trimethylstannyl or tributylstannyl compound is normally used. Although trimethylstannyl compounds show higher reactivity compared with tributylstannyl compounds, the toxicity of the former is about 1000 times larger than that of the latter. Therefore it is better to avoid using trimethylstannyl compounds unless necessary.
Several reviews have been published.
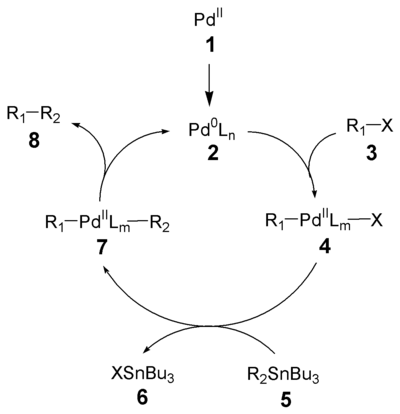
of the Stille reaction has been well studied. The first step in this catalytic cycle
is the reduction of the palladium
catalyst (1) to the active Pd(0) species (2). The oxidative addition
of the organohalide (3) gives a cis intermediate which rapidly isomerizes to the trans intermediate 4. Transmetalation
with the organostannane (5) forms intermediate 7, which produces the desired product (8) and the active Pd(0) species (2) after reductive elimination. The oxidative addition and reductive elimination retain the stereochemical configuration of the respective reactants.
 Rate of ligand transfer (transmetalation) from tin:
Rate of ligand transfer (transmetalation) from tin:
The low reactivity of alkyl stannanes is a serious drawback but can be remedied by the use of strongly polar solvents such as HMTP, DMF
or dioxane.
In 2007 the Stille reaction was subjected to a special type of mass spectrometry
allowing for the first time the direct experimental observation of a Pd(0)(PPh3)2 species (always assumed to exist but never before actually detected) and a cyclic transmetallation intermediate -Pd(II)-X-Sn-C- both through their radical cations.
is often added to the reaction mixture. This reagent stabilizes the intermediate complex formed by the oxidative addition of a catalyst and accelerates the reaction.
Reactivity and specificity of the Stille reaction can be improved by the addition of stoichiometric amounts of Cu(I)
or Mn(II)
salts.
The cross-coupling reaction can be inhibited by ligands of a high donor number
.
In the presence of Cu(I) salts, palladium-on-carbon has been shown to be an effective catalyst.
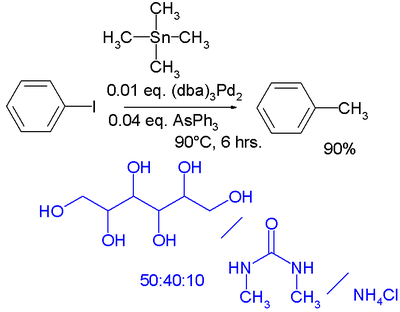
In the realm of green chemistry
a Stille reaction is reported taking place in a low melting and highly polar mixture of a sugar such as mannitol
, a urea
such as dimethylurea and a salt such as ammonium chloride
. The catalyst system is tris(dibenzylideneacetone)dipalladium(0)
with triphenylarsine
:
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
coupling an organotin compound with an sp2-hybridized organic halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
catalyzed by palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
. The reaction is widely used in organic synthesis
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
.

Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
, such as Cl
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, Br
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
, I
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
. Additionally, X can be a pseudohalide such as a triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
, C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
F
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
3S
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
3-.
The Stille reaction was discovered in 1977 by John Kenneth Stille
John Kenneth Stille
John Kenneth Stille was an American chemist who discovered the Stille reaction. He received B.A and M.A. degrees from the University of Arizona and his Ph.D. at the University of Illinois, where he met his wife-to-be Dolores Engelking...
and David Milstein
David Milstein
David Milstein is an Israeli chemist best known for his research on metal-mediated activation and functionalization of very strong chemical bonds.-Biography:...
, a post-doctorate in his laboratory. Stille reactions were used in 50% of all cross-coupling reactions published in 1992. The reaction continues to be exploited industrially, especially for pharmaceuticals.
The reaction is usually performed under inert atmosphere
Air-free technique
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen...
using dehydrated
Dehydration
In physiology and medicine, dehydration is defined as the excessive loss of body fluid. It is literally the removal of water from an object; however, in physiological terms, it entails a deficiency of fluid within an organism...
and degassed solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, as oxygen causes the oxidation of the palladium catalyst and promotes homo-coupling of organic stannyl
Organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849...
compounds, and these side reactions lead to a decrease in the yield of the desired cross-coupling reaction.
As the organic tin compound, a trimethylstannyl or tributylstannyl compound is normally used. Although trimethylstannyl compounds show higher reactivity compared with tributylstannyl compounds, the toxicity of the former is about 1000 times larger than that of the latter. Therefore it is better to avoid using trimethylstannyl compounds unless necessary.
Several reviews have been published.
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of the Stille reaction has been well studied. The first step in this catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
is the reduction of the palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
catalyst (1) to the active Pd(0) species (2). The oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
of the organohalide (3) gives a cis intermediate which rapidly isomerizes to the trans intermediate 4. Transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
with the organostannane (5) forms intermediate 7, which produces the desired product (8) and the active Pd(0) species (2) after reductive elimination. The oxidative addition and reductive elimination retain the stereochemical configuration of the respective reactants.

- alkynyl > alkenyl > aryl > allyl = benzyl > α-alkoxyalkyl > alkyl
The low reactivity of alkyl stannanes is a serious drawback but can be remedied by the use of strongly polar solvents such as HMTP, DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
or dioxane.
In 2007 the Stille reaction was subjected to a special type of mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
allowing for the first time the direct experimental observation of a Pd(0)(PPh3)2 species (always assumed to exist but never before actually detected) and a cyclic transmetallation intermediate -Pd(II)-X-Sn-C- both through their radical cations.
Variations
To improve the yield of the reaction, lithium chlorideLithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
is often added to the reaction mixture. This reagent stabilizes the intermediate complex formed by the oxidative addition of a catalyst and accelerates the reaction.
Reactivity and specificity of the Stille reaction can be improved by the addition of stoichiometric amounts of Cu(I)
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
or Mn(II)
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
salts.
The cross-coupling reaction can be inhibited by ligands of a high donor number
Donor number
In chemistry a donor number or DN is a qualitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 , in dilute solution in the noncoordinating solvent 1,2-dichloroethane with a...
.
In the presence of Cu(I) salts, palladium-on-carbon has been shown to be an effective catalyst.
In the realm of green chemistry
Green chemistry
Green chemistry, also called sustainable chemistry, is a philosophy of chemical research and engineering that encourages the design of products and processes that minimize the use and generation of hazardous substances...
a Stille reaction is reported taking place in a low melting and highly polar mixture of a sugar such as mannitol
Mannitol
Mannitol is a white, crystalline organic compound with the formula . This polyol is used as an osmotic diuretic agent and a weak renal vasodilator...
, a urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
such as dimethylurea and a salt such as ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
. The catalyst system is tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0)
Trisdipalladium or Pd23 is an organometallic complex based on palladium and dibenzylideneacetone used in organic chemistry. It was discovered in 1970.-Preparation and structure:...
with triphenylarsine
Triphenylarsine
Triphenylarsine is the chemical compound with the formula As3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis...
:

See also
- Organostannane additionOrganostannane additionOrganostannane addition reactions comprise the nucleophilic addition of an allyl-, allenyl-, or propargylstannane to an aldehyde, imine, or, in rare cases, a ketone....
- Heck reactionHeck reactionThe Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
- Hiyama couplingHiyama couplingIn organic chemistry, a Hiyama coupling is a palladium or nickel-catalyzed cross coupling reaction of organosilanes with organic halides or triflates. Hiyama couplings were first reported by Yasuo Hatanaka and Tamejiro Hiyama in 1988....
- Suzuki reactionSuzuki reactionThe Suzuki reaction is the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls, and has been extended to incorporate alkyl bromides...
- Palladium-catalyzed coupling reactionsPalladium-catalyzed coupling reactionsPalladium compounds are used as a catalyst in many coupling reactions, usually as a homogeneous catalyst. Examples include:* Heck reaction between alkenes and aryl halides* Suzuki reaction between aryl halides and boronic acids...
External links
- Stille reaction handout from the Myers group.
- Stille reaction at organic-chemistry.org
- Stille reaction - Synthetic protocols from organic-reaction.com

