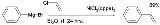
Kumada coupling
Encyclopedia
A Kumada coupling or Kumada-Corriu coupling is a cross coupling reaction in organic chemistry
between an alkyl or aryl
Grignard reagent and an aryl
or vinyl
halocarbon
catalysed by nickel
or palladium
. This reaction is relevant to organic synthesis
because it gives access to styrene derivatives. The reaction type was reported independently by two groups in 1972, and was named after Makoto Kumada
. The reaction forshadowed many related metal-catalyzed coupling reactions, such as the Sonogashira coupling
and the Suzuki coupling.
amounts of other metal halide catalysts than nickel based on silver
, copper
, and iron
. Stoichometric Grignard couplings and Grignard homo-couplings have been known well before that time.
The first report by the Kumada group described the reaction of a Grignard reagent for instance phenylmagnesium bromide
with an aryl or vinyl chloride such as vinyl chloride
to the coupled product (styrene) catalyzed by a nickel chloride complex dichloro(1,3-bis(diphenylphosphino)propane)nickel
.:
In the 1972 Corriu report, β-bromostyrene is allowed to react with phenylmagnesium bromide
to trans-stilbene
in diethylether also with nickel catalysts, notably nickel(II) acetylacetonate
.
Palladium was introduced to this chemistry in 1975 by Murahashi when tetrakis(triphenylphosphine)palladium(0)
was found to catalyze the reaction of (Z)-bromostyrene with methylmagnesium iodide to (Z)-propenylbenzene. With the far more reactive methyllithium, the palladium catalyst is not recycled fast enough and an elimination reaction
to the alkyne
predominates.
The reaction time was decreased from 24h to 20 minutes in 2001 by Haskwell et al. using a microreactor containing an immobilised nickel(II) catalyst.
for Ni(II) catalysts consists of a sequence of several steps :
The main steps in the mechanism for Ni(0) or Pd(0) catalysts are oxidative addition of the organohalide, transmetallation of the Grignard and reductive elimination.
tolerance :
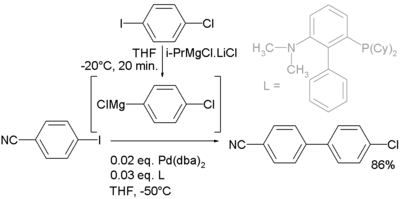
In this particular reaction the Grignard is prepared in situ
by I/Mg exchange between an aryl iodide and isopropylmagnesium chloride / lithium chloride
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
between an alkyl or aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
Grignard reagent and an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
or vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
halocarbon
Halocarbon
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds...
catalysed by nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
or palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
. This reaction is relevant to organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
because it gives access to styrene derivatives. The reaction type was reported independently by two groups in 1972, and was named after Makoto Kumada
Makoto Kumada
was a Japanese chemist and was a Professor of Chemistry first at Osaka City University and till his retirement in 1983 at Kyoto University in Japan. In 1972, Kumada's group reported nickel-catalyed cross coupling reactions nearly concurrently with the Corriu group working in France. The Kumada...
. The reaction forshadowed many related metal-catalyzed coupling reactions, such as the Sonogashira coupling
Sonogashira coupling
In organic chemistry, a Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides. This reaction was first reported by Kenkichi Sonogashira and Nobue Hagihara in 1975.-Catalyst:...
and the Suzuki coupling.
Development
This method builds on earlier work done by Tamura and Kochi in 1971 on couplings of Grignards with catalyticalCatalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
amounts of other metal halide catalysts than nickel based on silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
, copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, and iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
. Stoichometric Grignard couplings and Grignard homo-couplings have been known well before that time.
The first report by the Kumada group described the reaction of a Grignard reagent for instance phenylmagnesium bromide
Phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is so commonly used that it is commercially available as a solution in diethyl ether or tetrahydrofuran . Phenylmagnesium bromide is a Grignard reagent...
with an aryl or vinyl chloride such as vinyl chloride
Vinyl chloride
Vinyl chloride is the organochloride with the formula H2C:CHCl. It is also called vinyl chloride monomer, VCM or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride . At ambient pressure and temperature, vinyl chloride...
to the coupled product (styrene) catalyzed by a nickel chloride complex dichloro(1,3-bis(diphenylphosphino)propane)nickel
Dichloro(1,3-bis(diphenylphosphino)propane)nickel
Dichloro[1,3-bispropane]nickel a coordination complex with the formula NiCl2 . It is used as a catalyst in organic synthesis. The compound is an orange crystalline powder.-Structure and properties:...
.:
- C6H5MgBr + CH2=CHCl → C6H5CH=CH2 + MgBrCl
In the 1972 Corriu report, β-bromostyrene is allowed to react with phenylmagnesium bromide
Phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is so commonly used that it is commercially available as a solution in diethyl ether or tetrahydrofuran . Phenylmagnesium bromide is a Grignard reagent...
to trans-stilbene
Stilbene
-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining....
in diethylether also with nickel catalysts, notably nickel(II) acetylacetonate
Nickel(II) acetylacetonate
Nickel acetylacetonate is a coordination complex with the formula [Ni2]3. This dark green solid reacts with water to give the dihydrate Ni2·2H2O....
.
Palladium was introduced to this chemistry in 1975 by Murahashi when tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0)
Tetrakispalladium is the chemical compound Pd[P3]4, often abbreviated Pd4, or even PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.-Structure and properties:...
was found to catalyze the reaction of (Z)-bromostyrene with methylmagnesium iodide to (Z)-propenylbenzene. With the far more reactive methyllithium, the palladium catalyst is not recycled fast enough and an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
to the alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
predominates.
The reaction time was decreased from 24h to 20 minutes in 2001 by Haskwell et al. using a microreactor containing an immobilised nickel(II) catalyst.
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for Ni(II) catalysts consists of a sequence of several steps :
- Transmetallation: the dihalonickel catalyst (NiX2L2) reacts with the Grignard reagent to afford the diorganonickelOrganonickelOrganonickel chemistry is a branch of organometallic chemistry that deals with organic compounds feature nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic...
intermediate NiR2L2 and two equivalents of the dihalomagnesium salt 2 MgX2 - Oxidative additionOxidative additionOxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
: reaction of NiR2L2 with the organohalide R'X forms the coupled product R-R together with the nickelorganohalide NiR'XL2. In the overall reaction this step is negligible because the active nickel compound is formed in catalytical amounts - Transmetallation: in the first step of the catalytic cycleCatalytic cycleA catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
nickelorganohalide NiR'XL2 reacts with another equivalent of RMgX to mixed diorganonickel NiRR'L2 compound and dihalomagnesium MgX2 - trans-cis isomerization: with substrates trans-dichloroethylene and phenylmagnesium chloride, the resulting stilbene is enriched in the cis isomer.
- Coordination: a new equivalent of organohalide R'-X adds face-on to the mixed dihalonickel complex
- Reductive elimination: The cross-coupled product R-R' is released with regeneration of nickelorganohalide NiR'XL2
The main steps in the mechanism for Ni(0) or Pd(0) catalysts are oxidative addition of the organohalide, transmetallation of the Grignard and reductive elimination.
Scope
The scope of this reaction was extended to aryl-aryl couplings with improved functional groupFunctional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
tolerance :
In this particular reaction the Grignard is prepared in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
by I/Mg exchange between an aryl iodide and isopropylmagnesium chloride / lithium chloride
Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
.