Sigmatropic reaction
Encyclopedia
A sigmatropic reaction in organic chemistry
is a pericyclic reaction
wherein the net result is one σ-bond
is changed to another σ-bond in an uncatalyzed intramolecular
process. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon
-carbon bonds and the Greek word tropos, meaning turn. In this type of rearrangement reaction
, a substituent
moves from one part of a π-bonded system to another part in an intramolecular reaction with simultaneous rearrangement of the π system. True sigmatropic reactions are usually uncatalyzed, although Lewis acid
catalysis
is possible. Sigmatropic reactions often have transition-metal catalysts that form intermediates in analogous reactions. The most well-known of the sigmatropic rearrangements are the [3,3] Cope rearrangement
, Claisen rearrangement
, Carroll rearrangement
and the Fischer indole synthesis
.
s removed from the original location of the σ-bond. When the sum of i and j is an even number, this is an indication of the involvement of a neutral, all C atom chain. An odd number is an indication of the involvement of a charged C atom or of a heteroatom lone pair replacing a CC double bond. Thus, [1,5] and [3,3] shifts become [1,4] and [2,3] shifts with heteroatoms, while preserving symmetry considerations. Hydrogen
s are omitted in the third example for clarity.
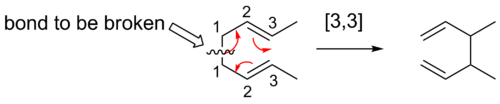
A convenient means of determining the order of a given sigmatropic rearrangement is to number the atoms of the bond being broken as atom 1, and then count the atoms in each direction from the broken bond to the atoms that form the new σ-bond in the product, numbering consecutively. The numbers that correspond to the atoms forming the new bond are then separated by a comma and placed within brackets to create the sigmatropic reaction order descriptor.
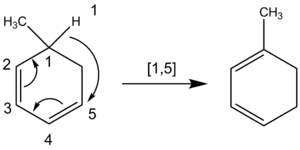
In the case of hydrogen atom migrations, a similar technique may be applied. When determining the order of a sigmatropic shift involving a hydrogen atom migration it is critical to count across all atoms involved in the reaction rather than only across the closest atoms. For example, the following hydrogen atom migration is of order [1,5], attained by counting counterclockwise through the π system, rather than the [1,3] order designation through the ring CH2 group that would mistakenly result if counted clockwise.
of the migrating atom or its other lobe is used to form the new bond.
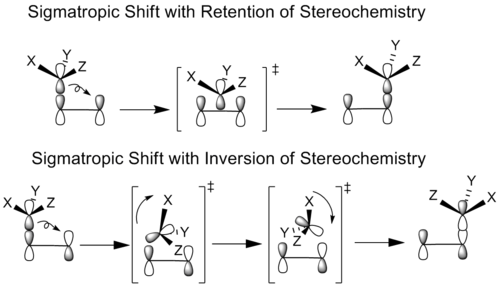
In cases of stereochemical
retention, the migrating group translates without rotation
into the bonding position, while in the case of stereochemical inversion the migrating group both rotates and translates to reach its bonded conformation.
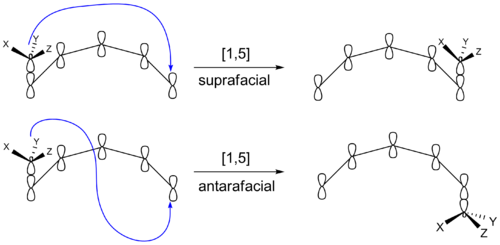
However, another stereochemical transition effect equally capable of producing inversion or retention products is whether the migrating group remains on the original face of the π system after rebonding or instead transfers to the opposite face of the π system. If the migrating group remains on the same face of the π system, the shift is known as suprafacial, while if the migrating group transfers to the opposite face is called an antarafacial shift, which are impossible for transformations that occur within small- or medium-sized rings.
[1,3] hydride
shift, a hydride moves three atoms. The Woodward-Hoffmann rules
dictate that it would proceed in an antarafacial shift. Although such a shift is symmetry allowed, the Mobius
topology
required in the transition state
prohibits such a shift because it is geometrically impossible, which accounts for the fact that enol
s do not isomerize without an acid
or base
catalyst.
molecules.
(i.e., have a diradical
mechanism, to which the Woodward-Hoffmann rules do not apply).
(-H
, -R or -Ar
) down 5 atoms of a π system. Hydrogen has been shown to shift in both cyclic and open chain systems at temperatures at or above 200 ˚C. These reactions are predicted to proceed suprafacially, via a Huckel-topology transition state.
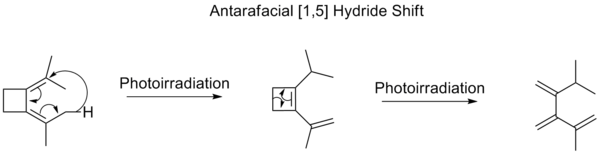
Photoirradiation would require an antarafacial shift of hydrogen. Although rare, there are examples where antarafacial shifts are favored:
In contrast to hydrogen [1,5] shifts, there have never been any observed [1,5] alkyl shifts in an open-chain system. Several studies have, however, been done to determine rate
preferences for [1,5] alkyl shifts in cyclic systems: carbonyl
and carboxyl> hydride> phenyl and vinyl
>> alkyl.
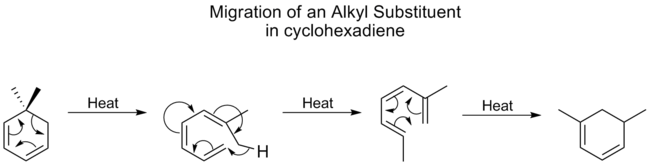
Alkyl groups undergo [1,5] shifts very poorly, usually requiring high temperatures, however, for cyclohexadienes
, the temperature for alkyl shifts isn’t much higher than that for carbonyls, the best migratory group. A study showed that this is because alkyl shifts on cyclohexadienes proceed through a different mechanism. First the ring opens, followed by a [1,7] shift, and then the ring reforms electrocyclically
:
This same mechanistic process is seen below, without the final electrocyclic ring-closing reaction, in the interconversion of lumisterol to vitamin D2.
to vitamin D2
, where following an electrocyclic ring opening to previtamin D2, a methyl hydrogen shifts.
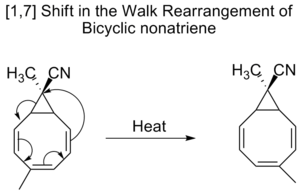
Bicyclic nonatrienes also undergo [1,7] shifts in a so called walk rearrangement, which is the shift of divalent
group, as part of a three-membered ring, in a bicyclic molecule
.
reactions would proceed suprafacially, via a Huckel topology transition state.
Discovered in 1912 by Rainer Ludwig Claisen
, the Claisen rearrangement is the first recorded example of a [3,3]-sigmatropic rearrangement. This rearrangement is a useful carbon
-carbon bond
-forming reaction
. An example of Claisen rearrangement is the [3,3] rearrangement of an allyl
vinyl
ether
, which upon heating yields a γ,δ-unsaturated carbonyl. The formation of a carbonyl group makes this reaction, unlike other sigmatropic rearrangements, inherently irreversible.

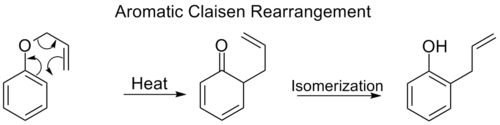
The ortho-Claisen rearrangement involves the [3,3] shift of an allyl
phenyl ether
to an intermediate which quickly tautomer
izes to an ortho-substituted phenol
.
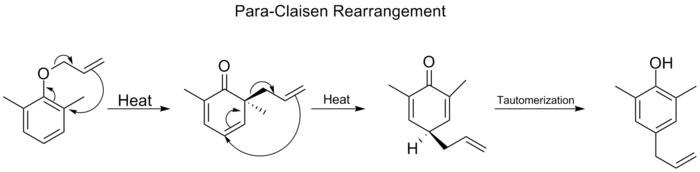
 When both the ortho
When both the ortho
positions on the benzene
ring are blocked, a second ortho-Claisen rearrangement will occur. This para-Claisen rearrangement ends with the tautomerization to a tri-substituted phenol.
The Cope rearrangement is an extensively studied organic reaction
involving the [3,3] sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope
. For example 3,4-dimethyl-1,5-hexadiene heated to 300 °C yields 2,6-octadiene.

The Oxy-Cope rearrangement a hydroxyl
group is added at C3 forming an enal or enone after Keto-enol tautomerism
of the intermediate enol:
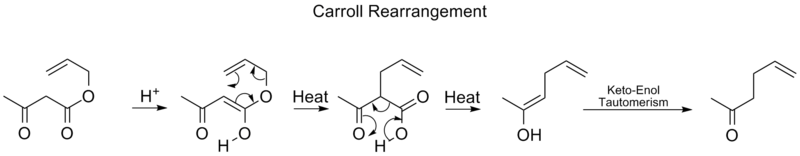
in organic chemistry
and involves the transformation of a β-keto
allyl
ester
into a α-allyl-β-ketocarboxylic acid. This organic reaction
is accompanied by decarboxylation
and the final product is a γ,δ-allylketone. The Carroll rearrangement is an adaptation of the Claisen rearrangement
and effectively a decarboxylative allylation.
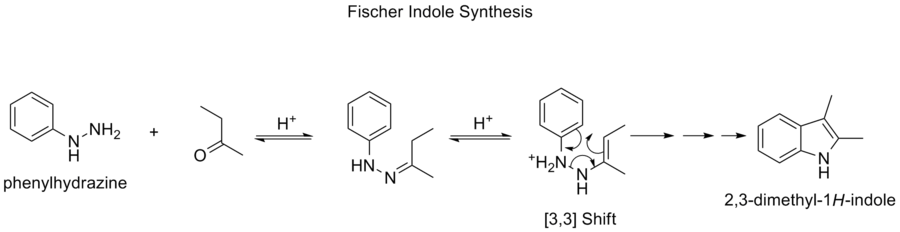
The Fischer indole synthesis is a chemical reaction
that produces the aromatic heterocycle
indole
from a (substituted) phenylhydrazine
and an aldehyde
or ketone
under acid
ic conditions. The reaction was discovered in 1883 by Hermann Emil Fischer
.
 The choice of acid catalyst is very important. Bronsted acids such as HCl
The choice of acid catalyst is very important. Bronsted acids such as HCl
, H2SO4
, polyphosphoric acid and p-toluenesulfonic acid
have been used successfully. Lewis acid
s such as boron trifluoride
, zinc chloride
, iron chloride
, and aluminium chloride
are also useful catalysts.
Several reviews have been published.
, S
, N
R or C
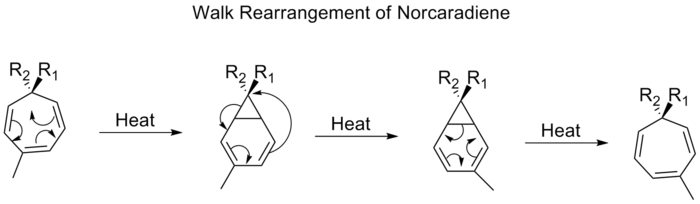
R2, which is part of a three-membered ring in a bicyclic molecule, is commonly referred to as a walk rearrangement. This can be formally characterized according to the Woodward-Hofmann rules as being a (1, n) sigmatropic shift. An example of such a rearrangement is the shift of substituents on tropilidenes (1,3,5-cycloheptatrienes). When heated, the pi-system goes through an electrocyclic ring closing to form bicycle[4,1,0]heptadiene (norcaradiene). Thereafter follows a [1,5] alkyl shift and an electrocyclic ring opening.
Proceeding through a [1,5] shift, the walk rearrangement of norcaradienes is expected to proceed suprafacially with a retention of stereochemistry. Experimental observations, however, show that the 1,5-shifts of norcaradienes proceed antarafacially. Theoretical calculations
found the [1,5] shift to be a diradical
process, but without involving any diradical minima on the potential energy surface
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a pericyclic reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
wherein the net result is one σ-bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
is changed to another σ-bond in an uncatalyzed intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
process. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-carbon bonds and the Greek word tropos, meaning turn. In this type of rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
, a substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
moves from one part of a π-bonded system to another part in an intramolecular reaction with simultaneous rearrangement of the π system. True sigmatropic reactions are usually uncatalyzed, although Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
is possible. Sigmatropic reactions often have transition-metal catalysts that form intermediates in analogous reactions. The most well-known of the sigmatropic rearrangements are the [3,3] Cope rearrangement
Cope rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope...
, Claisen rearrangement
Claisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
, Carroll rearrangement
Carroll rearrangement
The Carroll rearrangement is a rearrangement reaction in organic chemistry and involves the transformation of a β-keto allyl ester into a α-allyl-β-ketocarboxylic acid. This organic reaction is accompanied by decarboxylation and the final product is a γ,δ-allylketone...
and the Fischer indole synthesis
Fischer indole synthesis
The Fischer indole synthesis isa chemical reaction that produces the aromatic heterocycle indole from a phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Hermann Emil Fischer. Today antimigraine drugs of the triptan class are often...
.
Woodward-Hoffman Sigmatropic Shift Nomenclature
Sigmatropic rearrangements are concisely described by an order term [i,j], which is defined as the migration of a σ-bond adjacent to one or more π systems to a new position (i-1) and (j-1) atomAtom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s removed from the original location of the σ-bond. When the sum of i and j is an even number, this is an indication of the involvement of a neutral, all C atom chain. An odd number is an indication of the involvement of a charged C atom or of a heteroatom lone pair replacing a CC double bond. Thus, [1,5] and [3,3] shifts become [1,4] and [2,3] shifts with heteroatoms, while preserving symmetry considerations. Hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
s are omitted in the third example for clarity.
A convenient means of determining the order of a given sigmatropic rearrangement is to number the atoms of the bond being broken as atom 1, and then count the atoms in each direction from the broken bond to the atoms that form the new σ-bond in the product, numbering consecutively. The numbers that correspond to the atoms forming the new bond are then separated by a comma and placed within brackets to create the sigmatropic reaction order descriptor.
In the case of hydrogen atom migrations, a similar technique may be applied. When determining the order of a sigmatropic shift involving a hydrogen atom migration it is critical to count across all atoms involved in the reaction rather than only across the closest atoms. For example, the following hydrogen atom migration is of order [1,5], attained by counting counterclockwise through the π system, rather than the [1,3] order designation through the ring CH2 group that would mistakenly result if counted clockwise.
Suprafacial and Antarafacial Shifts
In principle, all sigmatropic shifts can occur with either a retention or inversion of the geometry of the migrating group, depending upon whether the original bonding lobeAtomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
of the migrating atom or its other lobe is used to form the new bond.
In cases of stereochemical
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
retention, the migrating group translates without rotation
Rotation
A rotation is a circular movement of an object around a center of rotation. A three-dimensional object rotates always around an imaginary line called a rotation axis. If the axis is within the body, and passes through its center of mass the body is said to rotate upon itself, or spin. A rotation...
into the bonding position, while in the case of stereochemical inversion the migrating group both rotates and translates to reach its bonded conformation.
However, another stereochemical transition effect equally capable of producing inversion or retention products is whether the migrating group remains on the original face of the π system after rebonding or instead transfers to the opposite face of the π system. If the migrating group remains on the same face of the π system, the shift is known as suprafacial, while if the migrating group transfers to the opposite face is called an antarafacial shift, which are impossible for transformations that occur within small- or medium-sized rings.
Thermal Hydride Shifts
In a thermalThermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation....
[1,3] hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
shift, a hydride moves three atoms. The Woodward-Hoffmann rules
Woodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
dictate that it would proceed in an antarafacial shift. Although such a shift is symmetry allowed, the Mobius
MOBIUS
MOBIUS is a consortium of libraries in Missouri, United States.The MOBIUS Consortium Office is a Missouri not-for-profit corporation established in 1998 by 50 libraries representing Missouri colleges and universities...
topology
Topology
Topology is a major area of mathematics concerned with properties that are preserved under continuous deformations of objects, such as deformations that involve stretching, but no tearing or gluing...
required in the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
prohibits such a shift because it is geometrically impossible, which accounts for the fact that enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
s do not isomerize without an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
or base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
catalyst.
Thermal Alkyl Shifts
Thermal alkyl [1,3] shifts, similar to [1,3] hydride shifts, must proceed antarafacially. Here the geometry of the transition state is prohibitive, but an alkyl group, due to the nature of its orbitals, can invert its geometry, form a new bond with the back lobe of its sp3 orbital, and therefore proceed via a suprafacial shift. These reactions are still not common in open chain systems because of the highly ordered nature of the transition state, which is more readily achieved in cyclicCyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
molecules.
Photochemical [1,3] Shifts
Photochemical [1,3] shifts should proceed through suprafacial shifts; however, most are non-concerted because they proceed through a triplet stateTriplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
(i.e., have a diradical
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
mechanism, to which the Woodward-Hoffmann rules do not apply).
[1,5] Shifts
A [1,5] shift involves the shift of 1 substituentSubstituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
(-H
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
, -R or -Ar
Aromatic hydrocarbon
An aromatic hydrocarbon or arene is a hydrocarbon with alternating double and single bonds between carbon atoms. The term 'aromatic' was assigned before the physical mechanism determining aromaticity was discovered, and was derived from the fact that many of the compounds have a sweet scent...
) down 5 atoms of a π system. Hydrogen has been shown to shift in both cyclic and open chain systems at temperatures at or above 200 ˚C. These reactions are predicted to proceed suprafacially, via a Huckel-topology transition state.
Photoirradiation would require an antarafacial shift of hydrogen. Although rare, there are examples where antarafacial shifts are favored:
In contrast to hydrogen [1,5] shifts, there have never been any observed [1,5] alkyl shifts in an open-chain system. Several studies have, however, been done to determine rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
preferences for [1,5] alkyl shifts in cyclic systems: carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
and carboxyl> hydride> phenyl and vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
>> alkyl.
Alkyl groups undergo [1,5] shifts very poorly, usually requiring high temperatures, however, for cyclohexadienes
1,3-Cyclohexadiene
1,3-Cyclohexadiene is a highly flammable cycloalkene that occurs as a colorless clear liquid. Its refractive index is 1.475 .It can be used as a hydrogen donor in transfer hydrogenation, since its conversion to benzene + hydrogen is in fact exothermic .Despite this apparent instability with respect...
, the temperature for alkyl shifts isn’t much higher than that for carbonyls, the best migratory group. A study showed that this is because alkyl shifts on cyclohexadienes proceed through a different mechanism. First the ring opens, followed by a [1,7] shift, and then the ring reforms electrocyclically
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
:
This same mechanistic process is seen below, without the final electrocyclic ring-closing reaction, in the interconversion of lumisterol to vitamin D2.
[1,7] Shifts
[1,7] sigmatropic shifts are predicted by the Woodward-Hoffmann rules to proceed in an antarafacial fashion, via a Mobius topology transition state. An antarafacial [1,7] shift is observed in the conversion of lumisterolLumisterol
Lumisterol is a compound that is part of the vitamin D family of steroid compounds. It is the stereoisomer of ergosterol and was produced as a photochemical by-product in the preparation of vitamin D1, which was a mixture of vitamin D2 and lumisterol. Vitamin D2 can be formed from lumisterol by a...
to vitamin D2
Ergocalciferol
Ergocalciferol is a form of vitamin D, also called vitamin D2. It is marketed under various names including Deltalin , Drisdol and Calcidol...
, where following an electrocyclic ring opening to previtamin D2, a methyl hydrogen shifts.
Bicyclic nonatrienes also undergo [1,7] shifts in a so called walk rearrangement, which is the shift of divalent
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
group, as part of a three-membered ring, in a bicyclic molecule
Bicyclic molecule
A bicyclic molecule is a molecule that features two fused rings. Bicyclic molecules occur widely in organic and inorganic compounds.Fusion of the rings can occur in three ways:...
.
[3,3] Shifts
[3,3] sigmatropic shifts are well studied sigmatropic rearrangements. The Woodward-Hoffman rules predict that these six electronElectron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
reactions would proceed suprafacially, via a Huckel topology transition state.
Claisen Rearrangement
Main article: Claisen rearrangementClaisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
Discovered in 1912 by Rainer Ludwig Claisen
Rainer Ludwig Claisen
Rainer Ludwig Claisen was a famous German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the university of Bonn , where he became a member of K.St.V. Arminia...
, the Claisen rearrangement is the first recorded example of a [3,3]-sigmatropic rearrangement. This rearrangement is a useful carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-carbon bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
-forming reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. An example of Claisen rearrangement is the [3,3] rearrangement of an allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
, which upon heating yields a γ,δ-unsaturated carbonyl. The formation of a carbonyl group makes this reaction, unlike other sigmatropic rearrangements, inherently irreversible.

Aromatic Claisen rearrangement
The ortho-Claisen rearrangement involves the [3,3] shift of an allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
phenyl ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
to an intermediate which quickly tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
izes to an ortho-substituted phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
.

Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
positions on the benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
ring are blocked, a second ortho-Claisen rearrangement will occur. This para-Claisen rearrangement ends with the tautomerization to a tri-substituted phenol.

Cope Rearrangement
Main article: Cope rearrangementCope rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope...
The Cope rearrangement is an extensively studied organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
involving the [3,3] sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope
Arthur C. Cope
Arthur C. Cope was a highly successful and influential organic chemist and member of the National Academy of Sciences. He is credited with the development of several important chemical reactions which bear his name including the Cope elimination and the Cope rearrangement.Cope was born on June...
. For example 3,4-dimethyl-1,5-hexadiene heated to 300 °C yields 2,6-octadiene.

Oxy-Cope rearrangement
The Oxy-Cope rearrangement a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group is added at C3 forming an enal or enone after Keto-enol tautomerism
Keto-enol tautomerism
In organic chemistry, keto-enol tautomerism refers to a chemical equilibrium between a keto form and an enol . The enol and keto forms are said to be tautomers of each other...
of the intermediate enol:
Carroll Rearrangement
The Carroll rearrangement is a rearrangement reactionRearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
and involves the transformation of a β-keto
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
into a α-allyl-β-ketocarboxylic acid. This organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
is accompanied by decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
and the final product is a γ,δ-allylketone. The Carroll rearrangement is an adaptation of the Claisen rearrangement
Claisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
and effectively a decarboxylative allylation.
Fischer Indole Synthesis
Main article: Fischer indole synthesisFischer indole synthesis
The Fischer indole synthesis isa chemical reaction that produces the aromatic heterocycle indole from a phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Hermann Emil Fischer. Today antimigraine drugs of the triptan class are often...
The Fischer indole synthesis is a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that produces the aromatic heterocycle
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
from a (substituted) phenylhydrazine
Phenylhydrazine
Phenylhydrazine is the chemical compound with the formula C6H5NHNH2. Organic chemists abbreviate the compound as PhNHNH2.- Chemical properties :...
and an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
under acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic conditions. The reaction was discovered in 1883 by Hermann Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
.

HCL
HCL or HCl can stand for:* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia* Hardware compatibility list...
, H2SO4
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, polyphosphoric acid and p-toluenesulfonic acid
P-Toluenesulfonic acid
p-Toluenesulfonic acid or tosylic acid is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as the Tosyl group and is often abbreviated as Ts or Tos...
have been used successfully. Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s such as boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
, zinc chloride
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
, iron chloride
Iron chloride
Iron chloride can refer to* Iron chloride , FeCl2* Iron chloride , FeCl3...
, and aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
are also useful catalysts.
Several reviews have been published.
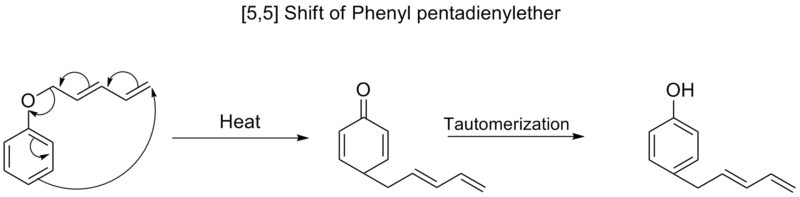
[5,5] Shifts
Similar to [3,3] shifts, the Woodward-Hoffman rules predict that [5,5] sigmatropic shifts would proceed suprafacially, Huckel topology transition state. These reactions are rarer than [3,3] sigmatropic shifts, but this is mainly a function of the fact that molecules that can undergo [5,5] shifts are rarer than molecules that can undergo [3,3] shifts.Walk Rearrangements
The migration of a divalent group, such as OOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, S
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
, N
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
R or C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
R2, which is part of a three-membered ring in a bicyclic molecule, is commonly referred to as a walk rearrangement. This can be formally characterized according to the Woodward-Hofmann rules as being a (1, n) sigmatropic shift. An example of such a rearrangement is the shift of substituents on tropilidenes (1,3,5-cycloheptatrienes). When heated, the pi-system goes through an electrocyclic ring closing to form bicycle[4,1,0]heptadiene (norcaradiene). Thereafter follows a [1,5] alkyl shift and an electrocyclic ring opening.
Proceeding through a [1,5] shift, the walk rearrangement of norcaradienes is expected to proceed suprafacially with a retention of stereochemistry. Experimental observations, however, show that the 1,5-shifts of norcaradienes proceed antarafacially. Theoretical calculations
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
found the [1,5] shift to be a diradical
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
process, but without involving any diradical minima on the potential energy surface
Potential energy surface
A potential energy surface is generally used within the adiabatic or Born–Oppenheimer approximation in quantum mechanics and statistical mechanics to model chemical reactions and interactions in simple chemical and physical systems...
.
See also
- 2,3-sigmatropic rearrangement2,3-sigmatropic rearrangement2,3-Sigmatropic rearrangements are a type of sigmatropic rearrangements and can be classified into two types. Rearrangements of allylic sulfoxides, amine oxides, selenoxides are neutral. Rearrangements of carbanions of allyl ethers are anionic. The general scheme for this kind of rearrangement...
- NIH shiftNIH shiftAn NIH shift is a chemical rearrangement where a hydrogen atom on an aromatic ring undergoes an intramolecular migration primarily during a hydroxylation reaction. This process is also known as a 1,2-hydride shift. These shifts are often studied and observed by isotopic labeling...
- Frontier Molecular Orbital TheoryFrontier Molecular Orbital TheoryIn chemistry, frontier molecular orbital theory is an application of MO theory describing HOMO / LUMO interactions.- History :In 1952, Kenichi Fukui published a paper in the Journal of Chemical Physics titled "A molecular theory of reactivity in aromatic hydrocarbons." Though widely criticized at...