
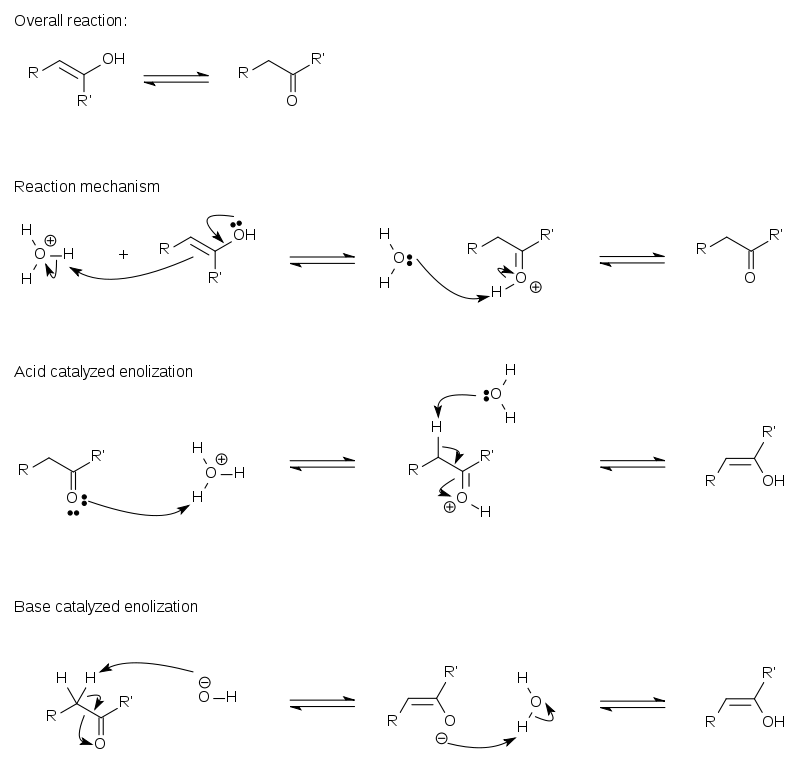
Keto-enol tautomerism
Encyclopedia
In organic chemistry
, keto-enol tautomerism refers to a chemical equilibrium
between a keto form (a ketone
or an aldehyde
) and an enol
(an alcohol
). The enol and keto forms are said to be tautomers of each other. The interconversion of the two forms involves the movement of a proton
and the shifting of bonding electron
s; hence, the isomerism qualifies as tautomerism.
A compound containing a carbonyl
group (C=O) is normally in rapid equilibrium
with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl
(−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important for some reactions. Furthermore, the deprotonated intermediate in the interconversion of the two forms, referred to as an enolate anion, is important in carbonyl chemistry, in large part because it is a strong nucleophile
.
Normally, the keto-enol tautomerization chemical equilibrium
is highly thermodynamically driven, and at room temperature the equilibrium heavily favors the formation of the keto form. A classic example for favoring the keto form can be seen in the equilibrium between vinyl alcohol and acetaldehyde
(K = [enol]/[keto] ≈ 3 x10−7). However, it is reported that in the case of vinyl alcohol, formation of a stabilized enol form can be accomplished by controlling the water concentration in the system and utilizing the kinetic favorability of the deuterium
produced kinetic isotope effect
(kH+/kD+ = 4.75, kH2O/kD2O = 12). Deuterium stabilization can be accomplished through hydrolysis of a ketene
precursor in the presence of a slight stoichiometric excess of heavy water
(D2O). Studies show that the tautomerization process is significantly inhibited at ambient temperatures ( kt ≈ 10−6 M/s), and the half life of the enol form can easily be increased to t1/2 = 42 minutes for first order hydrolysis kinetics.
in an aqueous acid
ic solution
.For this it is necessary that the alpha carbon(carbon closest to functional group) contains at least one hydrogen atom known as alpha hydrogen.This atom is removed from the alpha carbon and bonds to the oxygen of the carbonyl carbon to form the enol tautomer.
The existance of hydrogen atom at alpha carbon is necessary but not sufficient condition for enolization to occur. In order to be acidic the alpha hydrogen should be positioned such that may line up parallel with antibonding
pi-orbital of the carbonyl
group. The hyperconjugation
of this bond with C-H bond at alpha carbon reduces the electron density out of C-H bond and weakens it. Thus the alpha hydrogen becomes acidic. When this requirement is not enforced, for example in the adamantanone or other polycyclic ketones, the enolization is impossible or very slow. (J. E. Ordlander et al., Resistance of Adamantanone to Homoenolization, 1969), (J.B. Stothers and C.T. Tan,Adamantanone: Stereochemistry of its Homoenolization as shown by 2H Nuclear Magnetic Resonance, 1974)
First, the exposed electrons of the C=C double bond of the enol are donated to a hydronium ion (H3O+). This addition follows Markovnikov's rule
, thus the proton is added to the carbon with more hydrogens. This is a concerted step with the oxygen in the hydroxyl group donating electrons to produce the eventual carbonyl group.
Second, the oxygen in a water molecule donates electrons to the hydrogen in the hydroxyl group, thus relieving the positive charge on the electronegative oxygen atom.The keto form is more stable in single carbonyl carbon containing compounds as the carbon-oxygen pi bond is stronger and hence more stable than the carbon-carbon pi bond.
. His Erlenmeyer rule (developed in 1880) states that all alcohols in which the hydroxyl group is attached directly to a double-bonded carbon atom become aldehydes or ketones. This occurs because the keto form is, in general, more stable than its enol tautomer. As the lower energy form, the keto form is favored at equilibrium.
. The high phosphate-transfer potential of phosphoenolpyruvate
results from the fact that the phosphorylated compound is "trapped" in the less stable enol form, whereas after dephosphorylation it can assume the keto form. Rare enol tautomers of the bases guanine
and thymine
can lead to mutation because of their altered base-pairing properties .
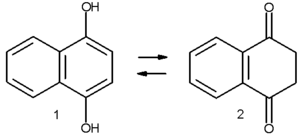
In certain aromatic compounds such as phenol
s, the enol is important due to the aromatic character of the enol but not the keto form. Melting the naphthalene
derivative 1,4-dihydroxynaphthalene 1 at 200 °C results in a 2:1 mixture with the keto form 2. Heating the keto form in benzene
at 120°C for three days also affords a mixture (1:1 with first-order reaction
kinetics). The keto product is kinetically stable and reverts back to the enol in presence of a base
. The keto form can be obtained in a pure form by stirring the keto form in trifluoroacetic acid
and toluene
(1:9 ratio) followed recrystallisation
from isopropyl ether.
 When the enol form is complexed
When the enol form is complexed
with chromium tricarbonyl, complete conversion to the keto form accelerated and occurs even at room temperature in benzene.
bases are in keto form. However, James Watson
and Francis Crick
first believed them to be in the enol tautomeric form, delaying the solution of the structure for several months.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, keto-enol tautomerism refers to a chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
between a keto form (a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
or an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
) and an enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
(an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
). The enol and keto forms are said to be tautomers of each other. The interconversion of the two forms involves the movement of a proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
and the shifting of bonding electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s; hence, the isomerism qualifies as tautomerism.
A compound containing a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group (C=O) is normally in rapid equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
(−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important for some reactions. Furthermore, the deprotonated intermediate in the interconversion of the two forms, referred to as an enolate anion, is important in carbonyl chemistry, in large part because it is a strong nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
.
Normally, the keto-enol tautomerization chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
is highly thermodynamically driven, and at room temperature the equilibrium heavily favors the formation of the keto form. A classic example for favoring the keto form can be seen in the equilibrium between vinyl alcohol and acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
(K = [enol]/[keto] ≈ 3 x10−7). However, it is reported that in the case of vinyl alcohol, formation of a stabilized enol form can be accomplished by controlling the water concentration in the system and utilizing the kinetic favorability of the deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
produced kinetic isotope effect
Kinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
(kH+/kD+ = 4.75, kH2O/kD2O = 12). Deuterium stabilization can be accomplished through hydrolysis of a ketene
Ketene
A ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
precursor in the presence of a slight stoichiometric excess of heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
(D2O). Studies show that the tautomerization process is significantly inhibited at ambient temperatures ( kt ≈ 10−6 M/s), and the half life of the enol form can easily be increased to t1/2 = 42 minutes for first order hydrolysis kinetics.
Mechanism
The conversion of an acid catalyzed enol to the keto form proceeds by a two step mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
in an aqueous acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
.For this it is necessary that the alpha carbon(carbon closest to functional group) contains at least one hydrogen atom known as alpha hydrogen.This atom is removed from the alpha carbon and bonds to the oxygen of the carbonyl carbon to form the enol tautomer.
The existance of hydrogen atom at alpha carbon is necessary but not sufficient condition for enolization to occur. In order to be acidic the alpha hydrogen should be positioned such that may line up parallel with antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
pi-orbital of the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group. The hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
of this bond with C-H bond at alpha carbon reduces the electron density out of C-H bond and weakens it. Thus the alpha hydrogen becomes acidic. When this requirement is not enforced, for example in the adamantanone or other polycyclic ketones, the enolization is impossible or very slow. (J. E. Ordlander et al., Resistance of Adamantanone to Homoenolization, 1969), (J.B. Stothers and C.T. Tan,Adamantanone: Stereochemistry of its Homoenolization as shown by 2H Nuclear Magnetic Resonance, 1974)
First, the exposed electrons of the C=C double bond of the enol are donated to a hydronium ion (H3O+). This addition follows Markovnikov's rule
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
, thus the proton is added to the carbon with more hydrogens. This is a concerted step with the oxygen in the hydroxyl group donating electrons to produce the eventual carbonyl group.
Second, the oxygen in a water molecule donates electrons to the hydrogen in the hydroxyl group, thus relieving the positive charge on the electronegative oxygen atom.The keto form is more stable in single carbonyl carbon containing compounds as the carbon-oxygen pi bond is stronger and hence more stable than the carbon-carbon pi bond.

Erlenmeyer rule
One of the early investigators into keto-enol tautomerism was Richard August Carl Emil ErlenmeyerRichard August Carl Emil Erlenmeyer
Richard August Carl Emil Erlenmeyer or Emil Erlenmeyer was a German chemist known for formulating the Erlenmeyer Ruleand designing a type of chemical flask.-Biography:...
. His Erlenmeyer rule (developed in 1880) states that all alcohols in which the hydroxyl group is attached directly to a double-bonded carbon atom become aldehydes or ketones. This occurs because the keto form is, in general, more stable than its enol tautomer. As the lower energy form, the keto form is favored at equilibrium.
Significance in biochemistry
Keto-enol tautomerism is important in several areas of biochemistryBiochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
. The high phosphate-transfer potential of phosphoenolpyruvate
Phosphoenolpyruvate
Phosphoenolpyruvic acid , or phosphoenolpyruvate as the anion, is an important chemical compound in biochemistry. It has the high-energy phosphate bond found in living organisms, and is involved in glycolysis and gluconeogenesis...
results from the fact that the phosphorylated compound is "trapped" in the less stable enol form, whereas after dephosphorylation it can assume the keto form. Rare enol tautomers of the bases guanine
Guanine
Guanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine . In DNA, guanine is paired with cytosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with...
and thymine
Thymine
Thymine is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. As the name suggests, thymine may be derived by methylation of uracil at...
can lead to mutation because of their altered base-pairing properties .
In certain aromatic compounds such as phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
s, the enol is important due to the aromatic character of the enol but not the keto form. Melting the naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
derivative 1,4-dihydroxynaphthalene 1 at 200 °C results in a 2:1 mixture with the keto form 2. Heating the keto form in benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
at 120°C for three days also affords a mixture (1:1 with first-order reaction
First-order reaction
First-order reaction may refer to:* Order of reaction, in chemical kinetics, the power to which the concentration term of a certain reactant in the rate equation is raised...
kinetics). The keto product is kinetically stable and reverts back to the enol in presence of a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
. The keto form can be obtained in a pure form by stirring the keto form in trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
and toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
(1:9 ratio) followed recrystallisation
Recrystallization (chemistry)
-Chemistry:In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted , which includes recrystallization...
from isopropyl ether.

Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
with chromium tricarbonyl, complete conversion to the keto form accelerated and occurs even at room temperature in benzene.
DNA
In deoxyribonucleic acids(DNA), the nucleotideNucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
bases are in keto form. However, James Watson
James D. Watson
James Dewey Watson is an American molecular biologist, geneticist, and zoologist, best known as one of the co-discoverers of the structure of DNA in 1953 with Francis Crick...
and Francis Crick
Francis Crick
Francis Harry Compton Crick OM FRS was an English molecular biologist, biophysicist, and neuroscientist, and most noted for being one of two co-discoverers of the structure of the DNA molecule in 1953, together with James D. Watson...
first believed them to be in the enol tautomeric form, delaying the solution of the structure for several months.

