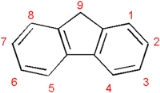
Polyfluorene
Encyclopedia
Polyfluorenes are a class of polymeric materials. They are of interest because similar to other conjugated polymers, they are currently being investigated for use in light-emitting diode
s, field-effect transistor
s, and plastic solar cells. They are not a naturally occurring material
, but are designed and synthesized for their applications. Modern chemistry has enabled adaptable synthesis and control over polyfluorenes that has facilitated use in many organic electronic
applications.
Academic and industrial research are interested in these polymers because of their optical and electrical properties
. They have high photoluminescence
quantum yields. They are a prototypical conjugated polymer but they are the only class of conjugated polymers which can be tuned to emit light
throughout the entire visible region. Polyfluorenes are primarily interesting because of the optoelectronic properties imbued by their chromophoric
constituents and their extended conjugation
. The design of polyfluorene derivatives relies on the character and properties of their monomer
s. Thus, the discovery and development of these polymeric repeat unit
s has had a profound influence on the development of polyfluorenes. The physical properties of polyfluorenes differ depending on their substitution pattern.
. Fluorene
, a principal repeat unit in polyfluorene derivatives, was isolated from coal tar
and discovered by Marcellin Berthelot
prior to 1883.
Its name originates from its interesting fluorescence
, fluorene became the subject of chemical-structure related color variation
(visible rather than luminescent), among other things, throughout the early to mid-20th century. Since it was an interesting chromophore
researchers wanted to understand which parts of the molecule were chemically reactive, and how substituting
these sites influenced the color. For instance, by adding various electron donating or electron accepting
moieties to fluorene, and by reacting with bases
, researchers were able to change the color of the molecule.
The physical properties of the fluorene molecule were recognizably desirable for polymers; as early as the 1970s researchers began incorporating this moiety into polymers. For instance, because of fluorene’s rigid, planar shape a polymer containing fluorene was shown to exhibit enhanced thermo-mechanical stability.
However, more promising was integrating the optoelectronic properties of fluorene into a polymer. Reports of the oxidative polymerization of fluorene (into a fully conjugated form) exist from at least 1972. However, it was not until after the highly publicized high conductivity of doped polyacetylene, presented in 1977 by Heeger, MacDiarmid and Shirakawa, that substantial interest in the electronic properties of conjugated polymers took off.
As interest in conducting plastics grew, fluorene again found application. The aromatic nature of fluorene makes it an excellent candidate component of a conducting polymer because it can stabilize and conduct
a charge; in the early 1980s fluorene was electropolymerized into conjugated polymer films with conductivities of 10−4 S cm−1.
The optical properties (such as variable luminescence
and visible light spectrum absorption) that accompany the extended conjugation in polymers of fluorene have become increasingly attractive for device applications. Throughout the 1990s and into the 2000s, many devices such as organic light-emitting diode
s (OLEDs), organic solar cell
s., organic thin film transistors, and biosensors have all taken advantage of the luminescent, electronic and absorptive properties of polyfluorenes.
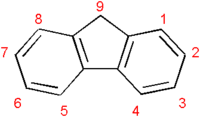 Polyfluorenes are an important class of polymers which have the potential to act as both electroactive and photoactive
Polyfluorenes are an important class of polymers which have the potential to act as both electroactive and photoactive
materials. This in part due to the shape of fluorene. Fluorene is generally planar; p-orbital overlap at the linkage between its two benzene
rings results in conjugation
across the molecule. This in turn allows for a reduced band gap as the excited state molecular orbitals are delocalized.
Since the degree of delocalization and the spatial location of the orbitals on the molecule is influenced by the electron donating (or withdrawing) character of its substituents, the band gap
energy can be varied. This chemical control over the band gap directly influences the color of the molecule by limiting the energies of light which it absorbs.
Interest in polyfluorene derivatives has increased because of their high photoluminescence quantum efficiency, high thermal stability, and their facile color tunability, obtained by introducing low-band-gap co-monomers. Research in this field has increased significantly due to its potential application in tuning organic light-emitting diode
s (OLEDs). In OLEDs, polyfluorenes are desirable because they are the only family of conjugated polymers that can emit colors spanning the entire visible range with high efficiency and low operating voltage. Furthermore, polyfluorenes are relatively soluble in most solvent
s, making them ideal for general applications.
Another important quality of polyfluorenes is their thermotropic
liquid crystal
linity which allows the polymers to align on rubbed polyimide
layers. Thermotropic liquid crystallinity refers to the polymers' ability to exhibit a phase transition
into the liquid crystal phase as the temperature is changed. This is very important to the development of liquid crystal display
s (LCDs) because the synthesis of liquid crystal displays requires that the liquid-crystal molecules at the two glass surfaces of the cell be aligned parallel to the two polarizer
foils.
This can only be done by coating the inner-surfaces of the cell with a thin, transparent
film of polyamide
which is then rubbed with a velvet cloth. Microscopic grooves are then generated in the polyamide layer and the liquid crystal in contact with the polyamide, the polyfluorene, can align in the rubbing direction. In addition to LCDs, polyfluorene can also be used to synthesize light emitting diodes
(LEDs). Polyfluorene has led to LEDs that can emit polarized light with polarization ratios of more than 20 and with brightness of 100 cd m−2. Even though this is very impressive, it is not sufficient for general applications.
and aggregate formation upon thermal annealing or when current is passed through them. Excimer formation involves the generation of dimerized units of the polymer which emit light at lower energies than the polymer itself. This hinders the use of polyfluorenes for most applications, including light-emitting diode
s (LED). When excimer or aggregate formation occurs this lowers the efficiency
of the LEDs by decreasing the efficiency of charge carrier recombination. Excimer formation also causes a red shift in the emission spectrum
.
Polyfluorenes can also undergo decomposition. There are two known ways in which decomposition can occur. The first involves the oxidation of the polymer that leads to the formation of an aromatic ketone, quenching the fluorescence. The second decomposition process results in aggregation leading to a red-shifted fluorescence, reduced intensity, exciton migration and relaxation through excimers.
Researchers have attempted to eliminate excimer formation and enhance the efficiency of polyfluorenes by copolymerizing polyfluorene with anthracene
and end-capping polyfluorenes with bulky groups which could sterically hinder excimer formation. Additionally, researchers have tried adding large substituents at the nine position of the fluorine in order to inhibit excimer and aggregate formation. Furthermore, researchers have tried to improve LEDs by synthesizing fluorene-triarylamine copolymers and other multilayer devices that are based on polyfluorenes that can be cross-linked. These have been found to have brighter fluorescence and reasonable efficiencies.
Aggregation has also been combated by varying the chemical structure. For example, when conjugated polymers aggregate, which is natural in the solid state, their emission can be self-quenched, reducing luminescent quantum yields and reducing luminescent device performance. In opposition to this tendency, researchers have used tri-functional monomers to create highly branched polyfluorenes which do not aggregate due to the bulkiness of the substituents. This design strategy has achieved luminescent quantum yields of 42% in the solid state.
This solution reduces the ease of processability of the material because branched polymers have increased chain entanglement and poor solubility.
Another problem commonly encountered by polyfluorenes is an observed broad green, parasitic emission which detracts from the color purity and efficiency needed for an OLED.
Initially attributed to excimer emission, this green emission has been shown to be due to the formation of ketone
defects along the fluorene polymer backbone (oxidation of the nine position on the monomer) when there are incomplete substitution at the nine positions of the fluorene monomer. Routes to combat this involve ensuring full substitution of the monomer’s active site, or including aromatic substituents. These solutions may present structures that lack optimal bulkiness or may be synthetically difficult.
Solubility
of the polymers are important because solution state processing is very common. Since conjugated polymers, with their planar structure, tend to aggregate, bulky side chain
s are added (to the 9 position of fluorene) to increase the solubility of the polymer.
of the polymers structure. However, oxidative polymerization does produce soluble polymers (from side-chain containing monomers) which are more easily characterized with nuclear magnetic resonance
.
polymers require regularly alternating electron donating and electron accepting monomers.
More recently, many popular cross-coupling chemistries have been applied to polyfluorenes and have enabled controlled polymerization; Palladium catalyzed cross couplings
such as Suzuki coupling
, Heck coupling
, etc., as well as nickel catalyzed Yamamoto and Grignard
coupling reactions have been applied to polymerization of fluorene derivatives. Such routes have enabled excellent control over the properties of polyfluorenes; the fluorene-thiophene-benzothiadiazole copolymer shown above, with a band gap of 1.78 eV when the side chains are alkoxy, appears blue because it is absorbing in the red wavelengths.
s (partly due to its fluorene monomer) excellent stability (due to its oxadiazole comonomer) good solubility (due to its many and branched alkyl side chains) and has an amine functionalized side chain for ease of tethering to other molecules or to a substrate.
The luminescent color of polyfluorenes can be changed, for example, (from blue to green-yellow) by adding functional groups which participate in excited state intramolecular proton transfer. Exchanging the alkoxy side chains for alcohol
side groups allows for energy dissipation (and a red-shift in emission) through reversible transfer of a proton from the alcohol to the nitrogen (on the oxadiazole). These complicated molecular structures were engineered to have these properties and were only able to be realized through careful control of their ordering and side group functionality.
monomers. Researchers at the Dow Chemical Company
synthesized several fluorene-based copolymers by alternating copolymerization using 5,5-dibromo-2,2-bithiophene which showed yellow emission and 4,7-dibromo-2,1,3-benzothiadiazole, which showed green emission. Other copolymerizations are also suitable; researchers at IBM
performed random copolymerization of fluorene with 3,9(10)-dibromoperylene,4,4-dibromo-R-cyanostilbene, and 1,4-bis(2-(4-bromophenyl)-1-cyanovinyl)-2-(2-ethylhexyl)-5-methoxybenzene. Only a small amount of the co-monomer, approximately 5%, was needed to tune the emission of the polyfluorene from blue to yellow. This example further illustrates that by introducing monomers that have a lower band gap than the fluorene monomer, one can tune the color that is emitted by the polymer.
Substitution at the nine position with various moieties has also been examined as a means to control the color emitted by polyfluorene. In the past researchers have tried putting alkyl substituents on the ninth position, however it has been found that by putting bulkier groups, such as alkoxyphenyl groups, the polymers had enhanced blue emission stability and superior polymer light-emitting diode performance (compared to polymers which have alkyl substituents at the ninth position).
s because of their affinity for property tuning. Copolymerization of fluorene with other monomers allows researchers to optimize the absorption and electronic energy levels as a means to increase the photovoltaic performance. For instance, by lowering the band gap of polyfluorenes, the absorption spectrum of the polymer can be adjusted to coincide with the maximum photon flux region of the solar spectrum
. This helps the solar cell absorb more of the sun's energy and to increase its energy conversion efficiency
; donor-acceptor structured copolymers of fluorene have achieved efficiencies above 4% when their absorption edge was pushed to 700 nm.
The voltage of polymer solar cells has also been increased through the design of polyfluorenes. These devices are typically produced by blending electron accepting and electron donating molecules which help separate charge to produce power. In polymer blend solar cells, the voltage produced by the device is determined by the difference between the electron donating polymer’s highest occupied molecular orbital (HOMO) energy level and the electron accepting molecules lowest unoccupied molecular orbital (LUMO) energy level. By adding electron withdrawing pendant molecules to conjugated polymers, their HOMO energy level can be lowered. For instance by adding electronegative groups on the end of conjugated side chains, researchers lowered the HOMO of a polyfluorene copolymer to −5.30 eV and increased the voltage of a solar cell to 0.99 V.
Typical polymer solar cells utilize fullerene
molecules as electron acceptors because of their low LUMO energy level (high electron affinity
). However the tunability of polyfluorenes allows their LUMO to be lowered to a level appropriate for use as an electron acceptor. Thus, polyfluorene copolymers have also been used in polymer:polymer blend solar cells, where their electron accepting, electron conducting and light absorbing properties enhance device performance.
Light-emitting diode
A light-emitting diode is a semiconductor light source. LEDs are used as indicator lamps in many devices and are increasingly used for other lighting...
s, field-effect transistor
Field-effect transistor
The field-effect transistor is a transistor that relies on an electric field to control the shape and hence the conductivity of a channel of one type of charge carrier in a semiconductor material. FETs are sometimes called unipolar transistors to contrast their single-carrier-type operation with...
s, and plastic solar cells. They are not a naturally occurring material
Natural material
A natural material is any product or physical matter that comes from plants, animals, or the ground. Minerals and the metals that can be extracted from them are also considered to belong into this category.* Biotic materials...
, but are designed and synthesized for their applications. Modern chemistry has enabled adaptable synthesis and control over polyfluorenes that has facilitated use in many organic electronic
Organic electronics
Organic electronics, plastic electronics or polymer electronics, is a branch of electronics dealing with conductive polymers, plastics, or small molecules. It is called 'organic' electronics because the polymers and small molecules are carbon-based...
applications.
Academic and industrial research are interested in these polymers because of their optical and electrical properties
Optoelectronics
Optoelectronics is the study and application of electronic devices that source, detect and control light, usually considered a sub-field of photonics. In this context, light often includes invisible forms of radiation such as gamma rays, X-rays, ultraviolet and infrared, in addition to visible light...
. They have high photoluminescence
Photoluminescence
Photoluminescence is a process in which a substance absorbs photons and then re-radiates photons. Quantum mechanically, this can be described as an excitation to a higher energy state and then a return to a lower energy state accompanied by the emission of a photon...
quantum yields. They are a prototypical conjugated polymer but they are the only class of conjugated polymers which can be tuned to emit light
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
throughout the entire visible region. Polyfluorenes are primarily interesting because of the optoelectronic properties imbued by their chromophoric
Chromophore
A chromophore is the part of a molecule responsible for its color. The color arises when a molecule absorbs certain wavelengths of visible light and transmits or reflects others. The chromophore is a region in the molecule where the energy difference between two different molecular orbitals falls...
constituents and their extended conjugation
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
. The design of polyfluorene derivatives relies on the character and properties of their monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s. Thus, the discovery and development of these polymeric repeat unit
Repeat unit
An essential concept which defines polymer structure, the repeat unit or repeating unit is a part of a polymer chain whose repetition would produce the complete polymer by linking the repeat units together successively along the chain, like the beads of a necklace.A repeat unit is sometimes called...
s has had a profound influence on the development of polyfluorenes. The physical properties of polyfluorenes differ depending on their substitution pattern.
History
Despite the similar sounding names, polyfluorene is unrelated to the element fluorineFluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
. Fluorene
Fluorene
Fluorene, or 9H-fluorene, is a polycyclic aromatic hydrocarbon. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It is combustible. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar...
, a principal repeat unit in polyfluorene derivatives, was isolated from coal tar
Coal tar
Coal tar is a brown or black liquid of extremely high viscosity, which smells of naphthalene and aromatic hydrocarbons. Coal tar is among the by-products when coal iscarbonized to make coke or gasified to make coal gas...
and discovered by Marcellin Berthelot
Marcellin Berthelot
Marcelin Pierre Eugène Berthelot was a French chemist and politician noted for the Thomsen-Berthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substances and disproved the theory of vitalism. He is considered as one of the greatest chemists of all time.He...
prior to 1883.
Its name originates from its interesting fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
, fluorene became the subject of chemical-structure related color variation
Pigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
(visible rather than luminescent), among other things, throughout the early to mid-20th century. Since it was an interesting chromophore
Chromophore
A chromophore is the part of a molecule responsible for its color. The color arises when a molecule absorbs certain wavelengths of visible light and transmits or reflects others. The chromophore is a region in the molecule where the energy difference between two different molecular orbitals falls...
researchers wanted to understand which parts of the molecule were chemically reactive, and how substituting
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
these sites influenced the color. For instance, by adding various electron donating or electron accepting
Polar effect
The Polar effect or electronic effect in chemistry is the effect exerted by a substituent on modifying electrostatic forces operating on a nearby reaction center...
moieties to fluorene, and by reacting with bases
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
, researchers were able to change the color of the molecule.
The physical properties of the fluorene molecule were recognizably desirable for polymers; as early as the 1970s researchers began incorporating this moiety into polymers. For instance, because of fluorene’s rigid, planar shape a polymer containing fluorene was shown to exhibit enhanced thermo-mechanical stability.
However, more promising was integrating the optoelectronic properties of fluorene into a polymer. Reports of the oxidative polymerization of fluorene (into a fully conjugated form) exist from at least 1972. However, it was not until after the highly publicized high conductivity of doped polyacetylene, presented in 1977 by Heeger, MacDiarmid and Shirakawa, that substantial interest in the electronic properties of conjugated polymers took off.
As interest in conducting plastics grew, fluorene again found application. The aromatic nature of fluorene makes it an excellent candidate component of a conducting polymer because it can stabilize and conduct
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
a charge; in the early 1980s fluorene was electropolymerized into conjugated polymer films with conductivities of 10−4 S cm−1.
The optical properties (such as variable luminescence
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
and visible light spectrum absorption) that accompany the extended conjugation in polymers of fluorene have become increasingly attractive for device applications. Throughout the 1990s and into the 2000s, many devices such as organic light-emitting diode
Organic light-emitting diode
An OLED is a light-emitting diode in which the emissive electroluminescent layer is a film of organic compounds which emit light in response to an electric current. This layer of organic semiconductor material is situated between two electrodes...
s (OLEDs), organic solar cell
Organic solar cell
An organic photovoltaic cell is a photovoltaic cell that uses organic electronics--a branch of electronics that deals with conductive organic polymers or small organic molecules for light absorption and charge transport....
s., organic thin film transistors, and biosensors have all taken advantage of the luminescent, electronic and absorptive properties of polyfluorenes.
Properties

Photoinduced electron transfer
Photoinduced electron transfer is an electron transfer which occurs when certain photoactive materials interact with light. - Breadth :Such materials include semiconductors that can be photoactivated like many solar cells, biological systems such as those used in photosynthesis, and small...
materials. This in part due to the shape of fluorene. Fluorene is generally planar; p-orbital overlap at the linkage between its two benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
rings results in conjugation
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
across the molecule. This in turn allows for a reduced band gap as the excited state molecular orbitals are delocalized.
Since the degree of delocalization and the spatial location of the orbitals on the molecule is influenced by the electron donating (or withdrawing) character of its substituents, the band gap
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
energy can be varied. This chemical control over the band gap directly influences the color of the molecule by limiting the energies of light which it absorbs.
Interest in polyfluorene derivatives has increased because of their high photoluminescence quantum efficiency, high thermal stability, and their facile color tunability, obtained by introducing low-band-gap co-monomers. Research in this field has increased significantly due to its potential application in tuning organic light-emitting diode
Organic light-emitting diode
An OLED is a light-emitting diode in which the emissive electroluminescent layer is a film of organic compounds which emit light in response to an electric current. This layer of organic semiconductor material is situated between two electrodes...
s (OLEDs). In OLEDs, polyfluorenes are desirable because they are the only family of conjugated polymers that can emit colors spanning the entire visible range with high efficiency and low operating voltage. Furthermore, polyfluorenes are relatively soluble in most solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s, making them ideal for general applications.
Another important quality of polyfluorenes is their thermotropic
Thermotropic
A liquid crystal is thermotropic if the order of its components is determined or changed by temperature.If temperature is too high, the rise in energy and therefore in motion of the components will induce a phase change: the LC will become an isotropic liquid.If, on the contrary, temperature is...
liquid crystal
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
linity which allows the polymers to align on rubbed polyimide
Polyimide
Polyimide is a polymer of imide monomers. The structure of imide is as shown. Polyimides have been in mass production since 1955...
layers. Thermotropic liquid crystallinity refers to the polymers' ability to exhibit a phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
into the liquid crystal phase as the temperature is changed. This is very important to the development of liquid crystal display
Liquid crystal display
A liquid crystal display is a flat panel display, electronic visual display, or video display that uses the light modulating properties of liquid crystals . LCs do not emit light directly....
s (LCDs) because the synthesis of liquid crystal displays requires that the liquid-crystal molecules at the two glass surfaces of the cell be aligned parallel to the two polarizer
Polarizer
A polarizer is an optical filter that passes light of a specific polarization and blocks waves of other polarizations. It can convert a beam of light of undefined or mixed polarization into a beam with well-defined polarization. The common types of polarizers are linear polarizers and circular...
foils.
This can only be done by coating the inner-surfaces of the cell with a thin, transparent
Transparency and translucency
In the field of optics, transparency is the physical property of allowing light to pass through a material; translucency only allows light to pass through diffusely. The opposite property is opacity...
film of polyamide
Polyamide
A polyamide is a polymer containing monomers of amides joined by peptide bonds. They can occur both naturally and artificially, examples being proteins, such as wool and silk, and can be made artificially through step-growth polymerization or solid-phase synthesis, examples being nylons, aramids,...
which is then rubbed with a velvet cloth. Microscopic grooves are then generated in the polyamide layer and the liquid crystal in contact with the polyamide, the polyfluorene, can align in the rubbing direction. In addition to LCDs, polyfluorene can also be used to synthesize light emitting diodes
Light-emitting diode
A light-emitting diode is a semiconductor light source. LEDs are used as indicator lamps in many devices and are increasingly used for other lighting...
(LEDs). Polyfluorene has led to LEDs that can emit polarized light with polarization ratios of more than 20 and with brightness of 100 cd m−2. Even though this is very impressive, it is not sufficient for general applications.
Challenges associated with polyfluorenes
Polyfluorenes often show both excimerExcimer
An excimer is a short-lived dimeric or heterodimeric molecule formed from two species, at least one of which is in an electronic excited state. Excimers are often diatomic and are composed of two atoms or molecules that would not bond if both were in the ground state. The lifetime of an excimer is...
and aggregate formation upon thermal annealing or when current is passed through them. Excimer formation involves the generation of dimerized units of the polymer which emit light at lower energies than the polymer itself. This hinders the use of polyfluorenes for most applications, including light-emitting diode
Light-emitting diode
A light-emitting diode is a semiconductor light source. LEDs are used as indicator lamps in many devices and are increasingly used for other lighting...
s (LED). When excimer or aggregate formation occurs this lowers the efficiency
Efficiency
Efficiency in general describes the extent to which time or effort is well used for the intended task or purpose. It is often used with the specific purpose of relaying the capability of a specific application of effort to produce a specific outcome effectively with a minimum amount or quantity of...
of the LEDs by decreasing the efficiency of charge carrier recombination. Excimer formation also causes a red shift in the emission spectrum
Emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted by the element's atoms or the compound's molecules when they are returned to a lower energy state....
.
Polyfluorenes can also undergo decomposition. There are two known ways in which decomposition can occur. The first involves the oxidation of the polymer that leads to the formation of an aromatic ketone, quenching the fluorescence. The second decomposition process results in aggregation leading to a red-shifted fluorescence, reduced intensity, exciton migration and relaxation through excimers.
Researchers have attempted to eliminate excimer formation and enhance the efficiency of polyfluorenes by copolymerizing polyfluorene with anthracene
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon consisting of three fused benzene rings. It is a component of coal-tar. Anthracene is used in the production of the red dye alizarin and other dyes...
and end-capping polyfluorenes with bulky groups which could sterically hinder excimer formation. Additionally, researchers have tried adding large substituents at the nine position of the fluorine in order to inhibit excimer and aggregate formation. Furthermore, researchers have tried to improve LEDs by synthesizing fluorene-triarylamine copolymers and other multilayer devices that are based on polyfluorenes that can be cross-linked. These have been found to have brighter fluorescence and reasonable efficiencies.
Aggregation has also been combated by varying the chemical structure. For example, when conjugated polymers aggregate, which is natural in the solid state, their emission can be self-quenched, reducing luminescent quantum yields and reducing luminescent device performance. In opposition to this tendency, researchers have used tri-functional monomers to create highly branched polyfluorenes which do not aggregate due to the bulkiness of the substituents. This design strategy has achieved luminescent quantum yields of 42% in the solid state.
This solution reduces the ease of processability of the material because branched polymers have increased chain entanglement and poor solubility.
Another problem commonly encountered by polyfluorenes is an observed broad green, parasitic emission which detracts from the color purity and efficiency needed for an OLED.
Initially attributed to excimer emission, this green emission has been shown to be due to the formation of ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
defects along the fluorene polymer backbone (oxidation of the nine position on the monomer) when there are incomplete substitution at the nine positions of the fluorene monomer. Routes to combat this involve ensuring full substitution of the monomer’s active site, or including aromatic substituents. These solutions may present structures that lack optimal bulkiness or may be synthetically difficult.
Synthesis and design
Conjugated polymers, such as polyfluorene, can be designed and synthesized with different properties for a wide variety of applications. The color of the molecules can be designed through synthetic control over the electron donating or withdrawing character of the substituents on fluorene or the comonomers in polyfluorene.Solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
of the polymers are important because solution state processing is very common. Since conjugated polymers, with their planar structure, tend to aggregate, bulky side chain
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
s are added (to the 9 position of fluorene) to increase the solubility of the polymer.
Oxidative polymerization
The earliest polymerizations of fluorene were oxidative polymerization with AlCl3 or FeCl3, and more commonly electropolymerization. Electropolymerization is an easy route to obtain thin, insoluble conducting polymer films. However, this technique has a few disadvantages in that does not provide controlled chain growth polymerizations, and processing and characterization are difficult as a result of its insolubility. Oxidative polymerization produces a similarly poor site-selectivity on the monomer for chain growth resulting in poor control over the regularityIsomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
of the polymers structure. However, oxidative polymerization does produce soluble polymers (from side-chain containing monomers) which are more easily characterized with nuclear magnetic resonance
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
.
Cross coupling polymerizations
The design of polymeric properties requires great control over the structure of the polymer. For instance, low band gapBand gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
polymers require regularly alternating electron donating and electron accepting monomers.
More recently, many popular cross-coupling chemistries have been applied to polyfluorenes and have enabled controlled polymerization; Palladium catalyzed cross couplings
Palladium-catalyzed coupling reactions
Palladium compounds are used as a catalyst in many coupling reactions, usually as a homogeneous catalyst. Examples include:* Heck reaction between alkenes and aryl halides* Suzuki reaction between aryl halides and boronic acids...
such as Suzuki coupling
Suzuki reaction
The Suzuki reaction is the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls, and has been extended to incorporate alkyl bromides...
, Heck coupling
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
, etc., as well as nickel catalyzed Yamamoto and Grignard
Kumada coupling
A Kumada coupling or Kumada-Corriu coupling is a cross coupling reaction in organic chemistry between an alkyl or aryl Grignard reagent and an aryl or vinyl halocarbon catalysed by nickel or palladium. This reaction is relevant to organic synthesis because it gives access to styrene derivatives...
coupling reactions have been applied to polymerization of fluorene derivatives. Such routes have enabled excellent control over the properties of polyfluorenes; the fluorene-thiophene-benzothiadiazole copolymer shown above, with a band gap of 1.78 eV when the side chains are alkoxy, appears blue because it is absorbing in the red wavelengths.
Design
Modern coupling chemistries allow other properties of polyfluorenes to be controlled through implementation of complex molecular designs. The above polymer structure pictured has excellent photoluminescent quantum yieldQuantum yield
The quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
s (partly due to its fluorene monomer) excellent stability (due to its oxadiazole comonomer) good solubility (due to its many and branched alkyl side chains) and has an amine functionalized side chain for ease of tethering to other molecules or to a substrate.
The luminescent color of polyfluorenes can be changed, for example, (from blue to green-yellow) by adding functional groups which participate in excited state intramolecular proton transfer. Exchanging the alkoxy side chains for alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
side groups allows for energy dissipation (and a red-shift in emission) through reversible transfer of a proton from the alcohol to the nitrogen (on the oxadiazole). These complicated molecular structures were engineered to have these properties and were only able to be realized through careful control of their ordering and side group functionality.
Organic light-emitting diodes (OLEDs)
In recent years many industrial efforts have focused on tuning the color of lights using polyfluorenes. It was found that by doping green or red emitting materials into polyfluorenes one could tune the color emitted by the polymers. Since polyfluorene homopolymers emit higher energy blue light, they can transfer energy via Förster resonance energy transfer (FRET) to lower energy emitters. In addition to doping, color of polyfluorenes can be tuned by copolymerizing the fluorene monomers with other low band gapBand gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
monomers. Researchers at the Dow Chemical Company
Dow Chemical Company
The Dow Chemical Company is a multinational corporation headquartered in Midland, Michigan, United States. As of 2007, it is the second largest chemical manufacturer in the world by revenue and as of February 2009, the third-largest chemical company in the world by market capitalization .Dow...
synthesized several fluorene-based copolymers by alternating copolymerization using 5,5-dibromo-2,2-bithiophene which showed yellow emission and 4,7-dibromo-2,1,3-benzothiadiazole, which showed green emission. Other copolymerizations are also suitable; researchers at IBM
IBM
International Business Machines Corporation or IBM is an American multinational technology and consulting corporation headquartered in Armonk, New York, United States. IBM manufactures and sells computer hardware and software, and it offers infrastructure, hosting and consulting services in areas...
performed random copolymerization of fluorene with 3,9(10)-dibromoperylene,4,4-dibromo-R-cyanostilbene, and 1,4-bis(2-(4-bromophenyl)-1-cyanovinyl)-2-(2-ethylhexyl)-5-methoxybenzene. Only a small amount of the co-monomer, approximately 5%, was needed to tune the emission of the polyfluorene from blue to yellow. This example further illustrates that by introducing monomers that have a lower band gap than the fluorene monomer, one can tune the color that is emitted by the polymer.
Substitution at the nine position with various moieties has also been examined as a means to control the color emitted by polyfluorene. In the past researchers have tried putting alkyl substituents on the ninth position, however it has been found that by putting bulkier groups, such as alkoxyphenyl groups, the polymers had enhanced blue emission stability and superior polymer light-emitting diode performance (compared to polymers which have alkyl substituents at the ninth position).
Polymer solar cells
Polyfluorenes are also used in polymer solar cellPolymer solar cell
Polymer solar cells are a type of flexible solar cell. They can come in many forms including: organic solar cell , or organic chemistry photovoltaic cell that produce electricity from sunlight using polymers. There are also other types of more stable thin-film semiconductors that can be deposited...
s because of their affinity for property tuning. Copolymerization of fluorene with other monomers allows researchers to optimize the absorption and electronic energy levels as a means to increase the photovoltaic performance. For instance, by lowering the band gap of polyfluorenes, the absorption spectrum of the polymer can be adjusted to coincide with the maximum photon flux region of the solar spectrum
Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
. This helps the solar cell absorb more of the sun's energy and to increase its energy conversion efficiency
Solar cell efficiency
The efficiency of a solar cell may be broken down into reflectance efficiency, thermodynamic efficiency, charge carrier separation efficiency and conductive efficiency...
; donor-acceptor structured copolymers of fluorene have achieved efficiencies above 4% when their absorption edge was pushed to 700 nm.
The voltage of polymer solar cells has also been increased through the design of polyfluorenes. These devices are typically produced by blending electron accepting and electron donating molecules which help separate charge to produce power. In polymer blend solar cells, the voltage produced by the device is determined by the difference between the electron donating polymer’s highest occupied molecular orbital (HOMO) energy level and the electron accepting molecules lowest unoccupied molecular orbital (LUMO) energy level. By adding electron withdrawing pendant molecules to conjugated polymers, their HOMO energy level can be lowered. For instance by adding electronegative groups on the end of conjugated side chains, researchers lowered the HOMO of a polyfluorene copolymer to −5.30 eV and increased the voltage of a solar cell to 0.99 V.
Typical polymer solar cells utilize fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
molecules as electron acceptors because of their low LUMO energy level (high electron affinity
Electron affinity
The Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
). However the tunability of polyfluorenes allows their LUMO to be lowered to a level appropriate for use as an electron acceptor. Thus, polyfluorene copolymers have also been used in polymer:polymer blend solar cells, where their electron accepting, electron conducting and light absorbing properties enhance device performance.

