
Chromophore
Encyclopedia
A chromophore is the part of a molecule
responsible for its color
.
The color arises when a molecule absorbs certain wavelength
s of visible light
and transmits or reflects others. The chromophore is a region in the molecule where the energy difference between two different molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron
from its ground state
into an excited state
.
In biology, molecules that serve to capture or detect light energy, the chromophore is the moiety
that causes a conformational change
of the molecule when hit by light.
(also known as resonating systems
) and metal complexes
.
, created by a series of alternating single and double bonds
, often in aromatic systems. Common examples include retinal
(used in the eye to detect light), various food coloring
s, fabric dyes (azo compound
s), pH indicator
s, lycopene
, β-carotene
, and anthocyanins. Various factors in a chromophore's structure go into determining at what wavelength region in a spectrum the chromophore will absorb. Lengthening or extending a conjugated system with more unsaturated (multiple) bonds in a molecule will tend to shift absorption to longer wavelengths. Woodward-Fieser rules can be used to approximate ultraviolet
-visible maximum absorption wavelength in organic compounds with conjugated pi-bond systems.
to ligands. Examples of such chromophores can be seen in chlorophyll
(used by plants for photosynthesis
), hemoglobin
, hemocyanin
, and colorful minerals such as malachite
and amethyst
.
A common motif in biochemistry is chromophores consisting of four pyrrole
rings. These come in two types:
is a functional group of atoms attached to a chromophore which modifies the ability of the chromophore to absorb light, altering the wavelength or intensity of the absorption.
occurs when a substance changes color as the pH
changes. This is a property of pH indicators, whose molecular structure
changes upon certain changes in the surrounding pH. This change in structure affects a chromophore in the pH indicator molecule. For example, phenolphthalein
is a pH indicator whose structure changes as pH changes as shown in the following table:
In a pH range of about 0-8, the molecule has three aromatic rings all bonded to a tetrahedral sp3 hybridized carbon atom in the middle which does not make the π-bonding in the aromatic rings conjugate. Because of their limited extent, the aromatic rings only absorb light in the ultraviolet region, and so the compound appears colorless in the 0-8 pH range. However as the pH increases beyond 8.2, that central carbon becomes part of a double bond becoming sp2 hybridized and leaving a p orbital to overlap with the π-bonding in the rings. This makes the three rings conjugate together to form an extended chromophore absorbing longer wavelength visible light to show a fuchsia color. At pH ranges outside 0-12, other molecular structure changes result in other color changes; see Phenolphthalein
for details.
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
responsible for its color
Color
Color or colour is the visual perceptual property corresponding in humans to the categories called red, green, blue and others. Color derives from the spectrum of light interacting in the eye with the spectral sensitivities of the light receptors...
.
The color arises when a molecule absorbs certain wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s of visible light
Visible spectrum
The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 750 nm. In terms of...
and transmits or reflects others. The chromophore is a region in the molecule where the energy difference between two different molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
from its ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
into an excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
.
In biology, molecules that serve to capture or detect light energy, the chromophore is the moiety
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
that causes a conformational change
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
of the molecule when hit by light.
Types of chromophore
Chromophores almost always arise in one of two forms: conjugated pi systemsConjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
(also known as resonating systems
Resonance
In physics, resonance is the tendency of a system to oscillate at a greater amplitude at some frequencies than at others. These are known as the system's resonant frequencies...
) and metal complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
.
Conjugated pi-bond system chromophores
In the conjugated chromophores, the electrons jump between energy levels that are extended pi orbitalsPi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
, created by a series of alternating single and double bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
, often in aromatic systems. Common examples include retinal
Retinal
Retinal, also called retinaldehyde or vitamin A aldehyde, is one of the many forms of vitamin A . Retinal is a polyene chromophore, and bound to proteins called opsins, is the chemical basis of animal vision...
(used in the eye to detect light), various food coloring
Food coloring
Food coloring is a substance, liquid or powder, that is added to food or drink to change its color. Food coloring is used both in commercial food production and in domestic cooking...
s, fabric dyes (azo compound
Azo compound
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
s), pH indicator
PH indicator
A pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions or hydrogen ions in the Arrhenius model. Normally, the indicator causes the...
s, lycopene
Lycopene
Lycopene is a bright red carotene and carotenoid pigment and phytochemical found in tomatoes and other red fruits and vegetables, such as red carrots, watermelons and papayas...
, β-carotene
Carotene
The term carotene is used for several related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but cannot be made by animals. Carotene is an orange photosynthetic pigment important for photosynthesis. Carotenes are all coloured to the human eye...
, and anthocyanins. Various factors in a chromophore's structure go into determining at what wavelength region in a spectrum the chromophore will absorb. Lengthening or extending a conjugated system with more unsaturated (multiple) bonds in a molecule will tend to shift absorption to longer wavelengths. Woodward-Fieser rules can be used to approximate ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
-visible maximum absorption wavelength in organic compounds with conjugated pi-bond systems.
Metal complex chromophores
The metal complex chromophores arise from the splitting of d-orbitals by binding of a transition metalTransition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
to ligands. Examples of such chromophores can be seen in chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
(used by plants for photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
), hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
, hemocyanin
Hemocyanin
Hemocyanins are respiratory proteins in the form of metalloproteins containing two copper atoms that reversibly bind a single oxygen molecule . Oxygenation causes a color change between the colorless Cu deoxygenated form and the blue Cu oxygenated form...
, and colorful minerals such as malachite
Malachite
Malachite is a copper carbonate mineral, with the formula Cu2CO32. This green-colored mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses. Individual crystals are rare but do occur as slender to acicular prisms...
and amethyst
Amethyst
Amethyst is a violet variety of quartz often used in jewelry. The name comes from the Ancient Greek ἀ a- and μέθυστος methustos , a reference to the belief that the stone protected its owner from drunkenness; the ancient Greeks and Romans wore amethyst and made drinking vessels of it in the belief...
.
A common motif in biochemistry is chromophores consisting of four pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
rings. These come in two types:
- the pyrroles form an open chain, no metal: phytochromePhytochromePhytochrome is a photoreceptor, a pigment that plants use to detect light. It is sensitive to light in the red and far-red region of the visible spectrum. Many flowering plants use it to regulate the time of flowering based on the length of day and night and to set circadian rhythms...
, phycobilinPhycobilinPhycobilins are chromophores found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads...
, bilirubinBilirubinBilirubin is the yellow breakdown product of normal heme catabolism. Heme is found in hemoglobin, a principal component of red blood cells. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases... - the pyrroles form a ring (porphyrinPorphyrinPorphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
), with a metal in the center: hemeHemeA heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
, chlorophyllChlorophyllChlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
Auxochrome
An auxochromeAuxochrome
An auxochrome is a group of atoms attached to a chromophore which modifies the ability of that chromophore to absorb light. Examples include the hydroxyl group , the amino group , and an aldehyde group ....
is a functional group of atoms attached to a chromophore which modifies the ability of the chromophore to absorb light, altering the wavelength or intensity of the absorption.
Halochromism in chromophores
HalochromismHalochromism
A halochromic material is a material which changes colour when pH changes occur. The term ‘chromic’ is defined as materials that can change colour reversibly with the presence of a factor. In this case, the factor is pH...
occurs when a substance changes color as the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
changes. This is a property of pH indicators, whose molecular structure
Molecular structure
The molecular structure of a substance is described by the combination of nuclei and electrons that comprise its constitute molecules. This includes the molecular geometry , the electronic properties of the...
changes upon certain changes in the surrounding pH. This change in structure affects a chromophore in the pH indicator molecule. For example, phenolphthalein
Phenolphthalein
Phenolphthalein is a chemical compound with the formula C20H14O4 and is often written as "HIn" or "phph" in shorthand notation. Often used in titrations, it turns colorless in acidic solutions and pink in basic solutions...
is a pH indicator whose structure changes as pH changes as shown in the following table:
| Structure | 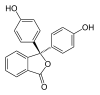 |
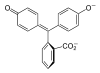 |
|---|---|---|
| pH | 0−8.2 | 8.2−12.0 |
| Conditions | acidic or near-neutral | basic |
| Color name | |
pink to fuchsia Fuchsia (color) Fuchsia is a vivid reddish or pinkish purple color named after the flower of the fuchsia plant, itself named after the German scientist Leonhart Fuchs... |
| Color | ||
In a pH range of about 0-8, the molecule has three aromatic rings all bonded to a tetrahedral sp3 hybridized carbon atom in the middle which does not make the π-bonding in the aromatic rings conjugate. Because of their limited extent, the aromatic rings only absorb light in the ultraviolet region, and so the compound appears colorless in the 0-8 pH range. However as the pH increases beyond 8.2, that central carbon becomes part of a double bond becoming sp2 hybridized and leaving a p orbital to overlap with the π-bonding in the rings. This makes the three rings conjugate together to form an extended chromophore absorbing longer wavelength visible light to show a fuchsia color. At pH ranges outside 0-12, other molecular structure changes result in other color changes; see Phenolphthalein
Phenolphthalein
Phenolphthalein is a chemical compound with the formula C20H14O4 and is often written as "HIn" or "phph" in shorthand notation. Often used in titrations, it turns colorless in acidic solutions and pink in basic solutions...
for details.
See also
- Visual phototransductionVisual phototransductionVisual phototransduction is a process by which light is converted into electrical signals in the rod cells, cone cells and photosensitive ganglion cells of the retina of the eye....
- Woodward's rulesWoodward's rulesWoodward's rules, named after Robert Burns Woodward and also known as Woodward-Fieser rules are several sets of empirically derived rules which attempt to predict the wavelength of the absorption maximum in an ultraviolet-visible spectrum of a given compound...
- ChromatophoreChromatophoreChromatophores are pigment-containing and light-reflecting cells found in amphibians, fish, reptiles, crustaceans, and cephalopods. They are largely responsible for generating skin and eye colour in cold-blooded animals and are generated in the neural crest during embryonic development...
- PigmentPigmentA pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
- PhotophorePhotophoreA photophore is a light-emitting organ which appears as luminous spots on various marine animals, including fish and cephalopods. The organ can be simple, or as complex as the human eye; equipped with lenses, shutters, color filters and reflectors...
- FluorophoreFluorophoreA fluorophore, in analogy to a chromophore, is a component of a molecule which causes a molecule to be fluorescent. It is a functional group in a molecule which will absorb energy of a specific wavelength and re-emit energy at a different wavelength...
External links
- Causes of Color: physical mechanisms by which color is generated.
- High Speed Nano-Sized Electronics May be Possible with Chromophores - Azonano.com

