
Thioether
Encyclopedia
Volatile organic compound
Volatile organic compounds are organic chemicals that have a high vapor pressure at ordinary, room-temperature conditions. Their high vapor pressure results from a low boiling point, which causes large numbers of molecules to evaporate or sublimate from the liquid or solid form of the compound and...
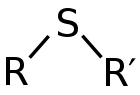
thioethers have foul odors. A thioether is similar to an ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
except that it contains a sulfur atom in place of the oxygen. Because oxygen and sulfur belong to the same group in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, the chemical properties of ethers and thioethers are somewhat similar.
Nomenclature
Thioethers are sometimes called sulfides, especially in the older literature and this term remains in use for the names of specific thioethers. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some thioethers are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisoleAnisole
Anisole, or methoxybenzene, is the organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances...
, C6H5OCH3.
Structure and properties
Thioether is an angular functional group, the C-S-C angle approaching 90°. The C-S bonds are about 180 pm.Thioethers are characterized by their strong odors, which are similar to thiol odor. This odor limits the applications of volatile thioethers. In terms of their physical properties they resemble ethers but are less volatile, higher melting, and less hydrophilic. These properties follow from the polarizability of the divalent sulfur center, which is greater than that for oxygen in ethers.
Thiophenes
ThiopheneThiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
s are a special class of thioether-containing heterocyclic compound
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
s. Because of their aromatic character, they are non-nucleophilic. The nonbonding electrons on sulfur are delocalized into the π-system. As a consequence, thiophene exhibits few properties expected for a thioether - thiophene is non-nucleophilic at sulfur and, in fact, is sweet-smelling. Upon hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
, thiophene gives tetrahydrothiophene
Tetrahydrothiophene
Tetrahydrothiophene is a heterocyclic organic compound consisting of a five-membered ring containing four carbon atoms and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, clear, colorless liquid with a strong unpleasant odor....
, C4H8S, which indeed does behave as a typical thioether.
Occurrence and applications
Thioethers are important in biology, notably in the amino acidAmino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
and the cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
biotin
Biotin
Biotin, also known as Vitamin H or Coenzyme R, is a water-soluble B-complex vitamin discovered by Bateman in 1916. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring...
. Petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
contains many organosulfur compounds, including thioethers. Polyphenylene sulfide is a useful high temperature plastic. Coenzyme M
Coenzyme M
Coenzyme M is a coenzyme required for methyl-transfer reactions in the metabolism of methanogens. The coenzyme is an anion with the formula . It is named 2-mercaptoethanesulfonate and abbreviated HS–CoM. The cation is unimportant, but the sodium salt is most available...
, CH3SCH2CH2SO3-, is the precursor to methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
(i.e. natural gas) via the process of methanogenesis
Methanogenesis
Methanogenesis or biomethanation is the formation of methane by microbes known as methanogens. Organisms capable of producing methane have been identified only from the domain Archaea, a group phylogenetically distinct from both eukaryotes and bacteria, although many live in close association with...
.
Preparation
Thioethers are typically prepared by the alkylationAlkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
s:
- R-Br + HS-R' → R-S-R' + HBr
Such reactions are usually conducted in the presence of base, which converts the thiol into the more nucleophilic thiolate. Analogously, the reaction of disulfide
Disulfide
In chemistry, a disulfide usually refers to the structural unit composed of a linked pair of sulfur atoms. Disulfide usually refer to a chemical compound that contains a disulfide bond, such as diphenyl disulfide, C6H5S-SC6H5....
s with organolithium reagent
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s produces thioethers:
- R3CLi + R1S-SR2 → R3CSR1 + R2SLi
Analogous reactions are known starting with Grignard reagents.
Alternatively, thioethers can be synthesized by the addition of a thiol to an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
:
- R-CH=CH2 + HS-R' → R-CH2-CH2-S-R'
This reaction is often catalysed by free radicals.
Thioethers can also be prepared by many other methods, such as the Pummerer rearrangement
Pummerer rearrangement
The Pummerer rearrangement is an organic reaction whereby an alkyl sulfoxide rearranges to an α-acyloxy–thioether in the presence of acetic anhydride. In this reaction, sulfur is reduced while adjacent carbon is oxidized....
. Trialkysulfonium salts react with nucleophiles with a dialkyl sulfide as a leaving group:
- Nu- + R3S+ → Nu-R + R-S-R
This reaction is exploited in biological systems as a means of transferring an alkyl group. For example, S-adenosylmethionine
S-Adenosyl methionine
S-Adenosyl methionine is a common cosubstrate involved in methyl group transfers. SAM was first discovered in Italy by G. L. Cantoni in 1952. It is made from adenosine triphosphate and methionine by methionine adenosyltransferase . Transmethylation, transsulfuration, and aminopropylation are the...
acts as a methylating agent
Methylation
In the chemical sciences, methylation denotes the addition of a methyl group to a substrate or the substitution of an atom or group by a methyl group. Methylation is a form of alkylation with, to be specific, a methyl group, rather than a larger carbon chain, replacing a hydrogen atom...
in biological SN2 reactions
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
.
Oxidation
While, in general, ethers are non-oxidizeable, thioethers can be easily oxidized to the sulfoxideSulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
s (R-S(=O)-R), which can themselves be further oxidized to sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
s (R-S(=O)2-R). Hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
is a typical oxidant. For example, dimethyl sulfide
Dimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
can be oxidized as follows:
- S(CH3)2 + H2O2 → OS(CH3)2Dimethyl sulfoxideDimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
+ H2O - OS(CH3)2 + H2O2 → O2S(CH3)2MethylsulfonylmethaneMethylsulfonylmethane is an organosulfur compound with the formula 2SO2. It is also known by several other names including DMSO2, methyl sulfone, and dimethyl sulfone. This colorless solid features the sulfonyl functional group and is considered relatively inert chemically...
+ H2O
Alkylation
Ethers can be alkylated at oxygen only with difficulty, but thioethers are readily alkylated to give stable sulfoniumSulfonium
A sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively charged ion featuring three organic substituents attached to sulfur. They have the formula [SR3]+...
salts, such as trimethylsulfonium iodide:
- S(CH3)2 + CH3I → [S(CH3)3]+I-
Binding to transition metals
In analogy to their easy alkylation, thioethers bind to metals to form coordination complexes. They are classified as soft ligands, but their affinity for metals is lower than typical phosphinePhosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
s. Chelating thioethers are known, such as 1,4,7-trithiacyclononane
1,4,7-Trithiacyclononane
1,4,7-Trithiacyclononane, also called 9-ane-S3, is the heterocyclic compound with the formula 3. This cyclic thioether is most often encountered as a tridentate ligand in coordination chemistry....
.
Hydrogenolysis
Thioethers undergo hydrogenolysisHydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
in the presence of certain metals:
- R-S-R' + 2 H2 → RH + R'H + H2S
Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
is useful for stoichiometric reactions in organic synthesis where as molybdenum-based catalysts are used to "sweeten" petroleum fractions, in the process called hydrodesulfurization
Hydrodesulfurization
Hydrodesulfurization is a catalytic chemical process widely used to remove sulfur from natural gas and from refined petroleum products such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils...
.

