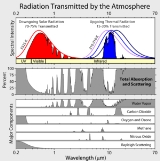
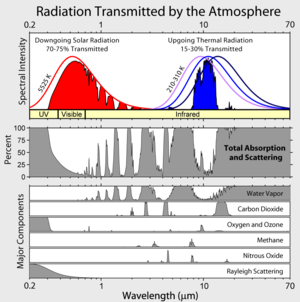
Absorption band
Encyclopedia
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s, frequencies
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
or energies in the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
which are able to excite a particular transition in a substance. Different types of absorption bands appear in spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, ranging from MHz radio frequency NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
and all kinds of traditional absorption spectroscopy
Absorption spectroscopy
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a...
techniques to γ-ray energy Mössbauer spectroscopy.
Overview
According to quantum mechanicsQuantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
, atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s and molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s can only hold certain defined quantities of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
, or exist in specific states. When electromagnetic radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
is absorbed by an atom or molecule, the energy of the radiation changes the state of the atom or molecule from an initial state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
to a final state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
. The number of states in a specific energy range is discrete for gaseous or diluted systems, with discrete energy level
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
s. Condensed systems
Condensed matter physics
Condensed matter physics deals with the physical properties of condensed phases of matter. These properties appear when a number of atoms at the supramolecular and macromolecular scale interact strongly and adhere to each other or are otherwise highly concentrated in a system. The most familiar...
, like fluids or solids, have a continuous density of states
Density of states
In solid-state and condensed matter physics, the density of states of a system describes the number of states per interval of energy at each energy level that are available to be occupied by electrons. Unlike isolated systems, like atoms or molecules in gas phase, the density distributions are not...
distribution and often possess continuous energy bands
Electronic band structure
In solid-state physics, the electronic band structure of a solid describes those ranges of energy an electron is "forbidden" or "allowed" to have. Band structure derives from the diffraction of the quantum mechanical electron waves in a periodic crystal lattice with a specific crystal system and...
. In order for a substance to change its energy it must do so in a series of "steps" by the absorption of a photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
. This absorption process can move a particle, like an electron, from an occupied state to an empty or unoccupied state. It can also move a whole vibrating or rotating system, like a molecule, from one vibrational or rotational state to another or it can create a quasiparticle
Quasiparticle
In physics, quasiparticles are emergent phenomena that occur when a microscopically complicated system such as a solid behaves as if it contained different weakly interacting particles in free space...
like a phonon
Phonon
In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, such as solids and some liquids...
or a plasmon
Plasmon
In physics, a plasmon is a quantum of plasma oscillation. The plasmon is a quasiparticle resulting from the quantization of plasma oscillations just as photons and phonons are quantizations of light and mechanical vibrations, respectively...
in a solid.
Electromagnetic transitions
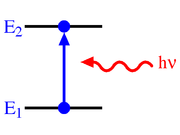
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
, momentum
Momentum
In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
, angular momentum
Angular momentum
In physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
, magnetic dipole moment and electric dipole moment are transported from the photon to the system. Because there are conservation law
Conservation law
In physics, a conservation law states that a particular measurable property of an isolated physical system does not change as the system evolves....
s, that have to be satisfied, the transition has to meet a series constraints. This results in a series of selection rules. It is not possible to make any transition that lies within the energy or frequency range that is observed.
The strength of an electromagnetic absorption process
Absorption cross section
Absorption cross section is a measure for the probability of an absorption process. More generally, the term cross section is used in physics to quantify the probability of a certain particle-particle interaction, e.g., scattering, electromagnetic absorption, etc...
is mainly determined by two factors. First it is important to realize that transitions that only change the magnetic dipole moment of the system are much weaker than transitions that change the electric dipole moment and that transitions to higher order moments like quadrupole transitions are weaker than dipole transitions. Second, not all transitions have the same transition matrix element, absorption coefficient or oscillator strength
Oscillator strength
An atom or a molecule can absorb light and undergo a transition fromone quantum state to another. The oscillator strength is a dimensionlessquantity to express the strength of the transition....
.
For some types of bands or spectroscopic disciplines temperature and statistical mechanics
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
plays an important role. For (far) infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
, microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
and radio frequency
Radio frequency
Radio frequency is a rate of oscillation in the range of about 3 kHz to 300 GHz, which corresponds to the frequency of radio waves, and the alternating currents which carry radio signals...
ranges the temperature dependent occupation numbers
Boltzmann distribution
In chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
of states and the difference between Bose-Einstein statistics
Bose–Einstein statistics
In statistical mechanics, Bose–Einstein statistics determines the statistical distribution of identical indistinguishable bosons over the energy states in thermal equilibrium.-Concept:...
and Fermi-Dirac statistics determines the intensity of observed absorptions. For other energy ranges thermal motion effects
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
, like Doppler broadening
Doppler broadening
In atomic physics, Doppler broadening is the broadening of spectral lines due to the Doppler effect caused by a distribution of velocities of atoms or molecules. Different velocities of the emitting particles result in different shifts, the cumulative effect of which is the line broadening.The...
may determine the linewidth
Spectral linewidth
The spectral linewidth characterizes the width of a spectral line, such as in the electromagnetic emission spectrum of an atom, or the frequency spectrum of an acoustic or electronic system...
.
Band and line shape
Cauchy distribution
The Cauchy–Lorentz distribution, named after Augustin Cauchy and Hendrik Lorentz, is a continuous probability distribution. As a probability distribution, it is known as the Cauchy distribution, while among physicists, it is known as the Lorentz distribution, Lorentz function, or Breit–Wigner...
or Gaussian, depending respectively on the decay mechanism
Relaxation
Relaxation stands quite generally for a release of tension, a return to equilibrium.In the sciences, the term is used in the following ways:*Relaxation , and more in particular:...
or temperature effects like Doppler broadening
Doppler broadening
In atomic physics, Doppler broadening is the broadening of spectral lines due to the Doppler effect caused by a distribution of velocities of atoms or molecules. Different velocities of the emitting particles result in different shifts, the cumulative effect of which is the line broadening.The...
. Analysis of the spectral density
Spectral density
In statistical signal processing and physics, the spectral density, power spectral density , or energy spectral density , is a positive real function of a frequency variable associated with a stationary stochastic process, or a deterministic function of time, which has dimensions of power per hertz...
and the intensities, width and shape of spectral line
Spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.- Types of line spectra :...
s sometimes can yield a lot of information about the observed system like it is done with Mössbauer spectra.
In systems with a very large number of states like macromolecule
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
s and large conjugated systems the separate energy levels can't always be distinguished in an absorption spectrum. If the line broadening mechanism is known and the shape of then spectral density is clearly visible in the spectrum, it is possible to get the desired data. Sometimes it is enough to know the lower or upper limits of the band or its position for an analysis.
For condensed matter
Condensed matter physics
Condensed matter physics deals with the physical properties of condensed phases of matter. These properties appear when a number of atoms at the supramolecular and macromolecular scale interact strongly and adhere to each other or are otherwise highly concentrated in a system. The most familiar...
and solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
s the shape of absorption bands are ofter determined by transitions between states in their continuous density of states
Density of states
In solid-state and condensed matter physics, the density of states of a system describes the number of states per interval of energy at each energy level that are available to be occupied by electrons. Unlike isolated systems, like atoms or molecules in gas phase, the density distributions are not...
distributions. For crystals
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
the electronic band structure
Electronic band structure
In solid-state physics, the electronic band structure of a solid describes those ranges of energy an electron is "forbidden" or "allowed" to have. Band structure derives from the diffraction of the quantum mechanical electron waves in a periodic crystal lattice with a specific crystal system and...
determines the density of states. In fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
s, glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
es and amorphous solid
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
s there is no long range correlation
Dynamic light scattering
thumb|right|350px|Hypothetical Dynamic light scattering of two samples: Larger particles on the top and smaller particle on the bottomDynamic light scattering is a technique in physics that can be used to determine the size distribution profile of small particles in suspension or polymers...
and the dispersion relation
Dispersion relation
In physics and electrical engineering, dispersion most often refers to frequency-dependent effects in wave propagation. Note, however, that there are several other uses of the word "dispersion" in the physical sciences....
s are isotropic. This makes density of states calculations of absorption band shapes easier. For charge-transfer complexes and conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
s the band width is determined by a variety of factors.
Electronic transitions
Electromagnetic transitions in atoms, molecules and condensed matter mainly take place at energies corresponding to the UV and visible part of the spectrum. Core electronCore electron
Core electrons are the electrons in an atom that are not valence electrons and therefore do not participate in bonding. An example: the carbon atom has a total of 6 electrons, 4 of them being valence electrons. So the remaining 2 electrons must be core electrons.They are so tightly bound to the...
s in atoms, and a lot of other phenomena, are observed with different brands of XAS in the X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
energy range. Electromagnetic transitions in atomic nuclei
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
, as observed in Mössbauer spectroscopy, take place in the gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
part of the spectrum. The main factors that cause broadening of the spectral line into an absorption band of a molecular solid are the distributions of vibrational and rotational energies of the molecules in the sample (and also those of their excited states). In solid crystals the shape of absorption bands are determined by the density of states
Density of states
In solid-state and condensed matter physics, the density of states of a system describes the number of states per interval of energy at each energy level that are available to be occupied by electrons. Unlike isolated systems, like atoms or molecules in gas phase, the density distributions are not...
of initial and final states of electronic states or lattice vibrations, called phonon
Phonon
In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, such as solids and some liquids...
s, in the crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
. In gas phase spectroscopy, the fine structure
Fine structure
In atomic physics, the fine structure describes the splitting of the spectral lines of atoms due to first order relativistic corrections.The gross structure of line spectra is the line spectra predicted by non-relativistic electrons with no spin. For a hydrogenic atom, the gross structure energy...
afforded by these factors can be discerned, but in solution-state spectroscopy, the differences in molecular micro environments further broaden the structure to give smooth bands. Electronic transition bands of molecules may be from tens to several hundred nanometers in breadth.
Vibrational transitions
Vibrational transitionsInfrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
and optical phonon transitions take place in the infrared part of the spectrum, at wavelengths of around 1-30 micrometres.
Rotational transitions
Rotational transitions take place in the far infrared and microwave regions.Other transitions
Absorption bands in the radio frequency range are found in NMR spectroscopyNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
. The frequency ranges and intensities are determined by the magnetic moment of the nuclei that are observed, the applied magnetic field and temperature occupation number differences of the magnetic states.
Applications
Materials with broad absorption bands are being applied in pigmentPigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
s, dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
s and optical filters
Filter (optics)
Optical filters are devices which selectively transmit light of different wavelengths, usually implemented as plane glass or plastic devices in the optical path which are either dyed in the mass or have interference coatings....
. Titanium dioxide, zinc oxide and chromophore
Chromophore
A chromophore is the part of a molecule responsible for its color. The color arises when a molecule absorbs certain wavelengths of visible light and transmits or reflects others. The chromophore is a region in the molecule where the energy difference between two different molecular orbitals falls...
s are applied as UV absorbers and reflectors in sunscreen
Sunscreen
Sunblock is a lotion, spray, gel or other topical product that absorbs or reflects some of the sun's ultraviolet radiation on the skin exposed to sunlight and thus helps protect against sunburn...
.
Absorption bands of interest to the atmospheric physicist
In oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
:
- the Hopfield bands, very strong, between about 67 and 100 nanometres in the ultraviolet (named after John J. Hopfield);
- a diffuse system between 101.9 and 130 nanometres;
- the Schumann-Runge continuum, very strong, between 135 and 176 nanometres;
- the Schumann-Runge bands between 176 and 192.6 nanometres (named for Victor SchumannVictor SchumannVictor Schumann was a physicist and spectroscopist who in 1893 discovered the vacuum ultraviolet.He was the first to measure spectra below 200 nm. For this, he used a prism and lenses in fluorin instead of quartz, he prepared himself photographic plates with a reduced layer of gelatin, and he...
and Carl RungeCarle David Tolmé RungeCarl David Tolmé Runge was a German mathematician, physicist, and spectroscopist.He was co-developer and co-eponym of the Runge–Kutta method , in the field of what is today known as numerical analysis.-Biography:...
); - the Herzberg bands between 240 and 260 nanometres (named after Gerhard HerzbergGerhard HerzbergGerhard Heinrich Friedrich Otto Julius Herzberg, was a pioneering physicist and physical chemist, who won the Nobel Prize for Chemistry in 1971, "for his contributions to the knowledge of electronic structure and geometry of molecules, particularly free radicals". Herzberg's main work concerned...
); - the atmospheric bands between 538 and 771 nanometres in the visible spectrum; and
- a system in the infrared at about 1000 nanometres.
In ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
:
- the Hartley bands between 200 and 300 nanometres in the ultraviolet, with a very intense maximum absorption at 255 nanometres (named after Walter Noel Hartley);
- the Huggins bands, weak absorption between 320 and 360 nanometres (named after Sir William HugginsWilliam HugginsSir William Huggins, OM, KCB, FRS was an English amateur astronomer best known for his pioneering work in astronomical spectroscopy.-Biography:...
); - the Chappuis bands (sometimes misspelled "Chappius"), a weak diffuse system between 375 and 650 nanometres in the visible spectrum (named after J. Chappuis); and
- the Wulf bands in the infrared beyond 700 nm, centered at 4,700, 9,600 and 14,100 nanometres, the latter being the most intense (named after Oliver R. Wulf).
In nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
:
- The Lyman-Birge-Hopfield bands, sometimes known as the Birge-Hopfield bands, in the far ultraviolet: 140– 170 nm (named after Theodore LymanTheodore LymanTheodore Lyman was a U.S. physicist and spectroscopist, born in Boston. He graduated from Harvard in 1897, from which he also received his Ph.D. in 1900. He became an assistant professor in physics at Harvard, where he remained, becoming full professor in 1917, and where he was also director of...
, Raymond T. Birge, and John J. Hopfield)
See also
- Franck-Condon principleFranck-Condon principleThe Franck–Condon principle is a rule in spectroscopy and quantum chemistry that explains the intensity of vibronic transitions. Vibronic transitions are the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the...
- SpectroscopySpectroscopySpectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
- Spectral lineSpectral lineA spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.- Types of line spectra :...

