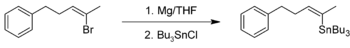Organotin
Encyclopedia
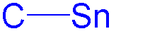
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
with hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
substituents. Organotin chemistry is part of the wider field of organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland
Edward Frankland
Sir Edward Frankland, KCB, FRS was a chemist, one of the foremost of his day. He was an expert in water quality and analysis, and originated the concept of combining power, or valence, in chemistry. He was also one of the originators of organometallic chemistry.-Biography:Edward Frankland was born...
in 1849. An organotin compound is commercially applied as a hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
scavenger
Scavenger (chemistry)
A scavenger in chemistry is a chemical substance added to a mixture in order to remove or inactivate impurities or unwanted reaction products. Their use is wide-ranged:...
(or heat stabilizer) in polyvinyl chloride
Polyvinyl chloride
Polyvinyl chloride, commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups having one hydrogen replaced by chloride. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene. PVC is widely used in...
and as a biocide
Biocide
A biocide is a chemical substance or microorganism which can deter, render harmless, or exert a controlling effect on any harmful organism by chemical or biological means. Biocides are commonly used in medicine, agriculture, forestry, and industry...
. Tributyltin oxide
Tributyltin oxide
Tributyltin oxide , or bisoxide, is an organotin compound chiefly used as a biocide , especially a wood preservative. Its chemical formula is C24H54OSn2. It has the form of a thin, colorless to pale yellow liquid with melting point -45 °C, boiling point 180 °C, and slight water solubility...
has been extensively used as a wood preservative. Tributyltin compounds are used as marine anti-biofouling
Biofouling
Biofouling or biological fouling is the undesirable accumulation of microorganisms, plants, algae, or animals on wetted structures.-Impact:...
agents. Concerns over toxicity of these compounds (some reports describe biological effects to marine life at a concentration of 1 nanogram per liter) have led to a worldwide ban by the International Maritime Organization
International Maritime Organization
The International Maritime Organization , formerly known as the Inter-Governmental Maritime Consultative Organization , was established in Geneva in 1948, and came into force ten years later, meeting for the first time in 1959...
. n-Butyltin trichloride is used in the production of tin dioxide
Tin dioxide
Tin dioxide is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names , this oxide of tin is the most important raw material in tin chemistry...
layers on glass bottles by chemical vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
.
Preparation of organotin compounds
Organotin compounds can be synthesised by reaction of a Grignard reagent with tin halides for example tin tetrachloride. An example is the organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of tributyl-[(Z)-5-phenyl-2-penten-2-yl]stannane:
The Wurtz-like
Wurtz reaction
The Wurtz reaction, named after Charles-Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium to form a new carbon-carbon bond:...
coupling of alkyl sodium compounds with tin halides yield tetraorganotin compounds. Another method is an exchange reaction of tin halides with organoaluminium compounds (AlR3). Triorganotin halides can be prepared in the Kocheshkov redistribution reaction.
Reactions of organotin compounds
Important reactions involving organotin compounds are the Stille reactionStille reaction
The Stille reaction is a chemical reaction coupling an organotin compound with an sp2-hybridized organic halide catalyzed by palladium. The reaction is widely used in organic synthesis....
(coupling reaction with sp2-hybridized organic halides catalyzed by palladium):
and organostannane addition
Organostannane addition
Organostannane addition reactions comprise the nucleophilic addition of an allyl-, allenyl-, or propargylstannane to an aldehyde, imine, or, in rare cases, a ketone....
s (nucleophilic addition of an allyl-, allenyl-, or propargylstannanes to an aldehydes and imines). Organotin compounds are also used extensively in radical chemistry
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
(eg. radical cyclizations
Radical cyclization
Radical cyclization reactions are organic chemical transformations that yield cyclic products via radical intermediates. They usually proceed in three basic steps: selective radical generation, radical cyclization, and conversion of the cyclized radical to product.-Introduction:Radical cyclization...
, Barton–McCombie deoxygenation, Barton decarboxylation
Barton decarboxylation
The Barton decarboxylation is a radical reaction in which a carboxylic acid is first converted to a thiohydroxamate ester . The product is then heated in the presence of a radical initiator and a suitable hydrogen donor to complete the reductive decarboxylation of the initial carboxylic acid...
, etc.).
Use and toxicity
- Tetraorganotins are very stable molecules with low toxicity and low biological activity. They are unusable as biocides, but they can be metabolized to toxic triorganotin compounds. They are used as starting materials for catalysts.
- Triorganotins are very toxic. Tri-n-alkyltins are phytotoxic and therefore cannot be used in agriculture. Depending on the organic groups, they can be powerful bactericides and fungicideFungicideFungicides are chemical compounds or biological organisms used to kill or inhibit fungi or fungal spores. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality and profit. Fungicides are used both in agriculture and to fight fungal infections in animals...
s. TributyltinTributyltinTributyltin compounds are a group of compounds containing the 3Sn moiety, such as tributyltin hydride or tributyltin oxide. They are the main active ingredients in certain biocides used to control a broad spectrum of organisms...
s are used as industrial biocides, e.g. as antifungal agents in textiles and paper, wood pulp and paper mill systems, breweries, and industrial cooling systems. Tributyltins are also used in marine anti-fouling paint. Triphenyltins are used as active components of antifungal paints and agricultural fungicides. Other triorganotins are used as miticideMiticideAcaricides are pesticides that kill members of the Acari group, which includes ticks and mites.Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields.-Terminology:...
s and acaricides. - Diorganotins have no antifungal activity, low toxicity, and low antibacterial activity, except for diphenyltins. They are used in polymer manufacturing, as PVCPolyvinyl chloridePolyvinyl chloride, commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups having one hydrogen replaced by chloride. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene. PVC is widely used in...
heat stabilizers, catalysts, in the manufacturing of polyurethanePolyurethaneA polyurethane is any polymer composed of a chain of organic units joined by carbamate links. Polyurethane polymers are formed through step-growth polymerization, by reacting a monomer with another monomer in the presence of a catalyst.Polyurethanes are...
and siliconeSiliconeSilicones are inert, synthetic compounds with a variety of forms and uses. Typically heat-resistant and rubber-like, they are used in sealants, adhesives, lubricants, medical applications , cookware, and insulation....
curing. DBTDibutyltin oxideDibutyltin oxide, or dibutyloxotin, is an organotin compound used in organic synthesis. Among many synthetic applications, it is particularly useful in regioselective alkylation, acylation, and sulfonation reactions for starting materials containing alcohol functional groups.- Applications...
is however immunotoxic, and a recent paper suggests a link to auto-immune related diseases. - Monoorganotins have no biocidal activity and their toxicity to mammals is very low. Methyltin, butyltin, octyltin and monoestertins are used as PVC heat stabilizers.
- Many different organotin complexes are being studied in anticancer therapy, observing that their cytotoxicity and selectivity towards cancer cell is higher than that of cisplatinCisplatinCisplatin, cisplatinum, or cis-diamminedichloroplatinum is a chemotherapy drug. It is used to treat various types of cancers, including sarcomas, some carcinomas , lymphomas, and germ cell tumors...
.
Compounds
Organotin compounds are used commercially in a wide range of applications such as biocides, insecticides, chemical intermediates and as catalysts.- Tributyltin azideTributyltin azideTributyltin azide is an organotin compound. It is usually synthesized from tributyltin chloride and sodium azide.-Uses:Tributyltin azide is a reagent used in the synthesis of tetrazolylbenzene compounds...
Other classifications
PolystannanePolystannane
Polystannanes are organotin compounds with the formula n. These polymers have been of intermitant academic interest; they are unusual because heavy elements comprise the backbone...
s are polymeric stannanes of the type (SnR2)n
Stannole
Stannole
Stannole is a organotin compound with the formula 4SnH2. It is classified as a metallole, i.e. an unsaturated five-membered ring containing a heteroatom. It is a structural analog of pyrrole, with tin replacing the nitrogen...
s are the structural analogs of pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
. Unsaturated organostannanes also exist: stannenes are compounds of the type RRC=SnRR with a formal double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
(also see stannabenzene
Stannabenzene
Stannabenzene is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom. Stannabenzene itself has been studied by computational chemistry, but has not been isolated....
) and distannenes have a tin to tin double bond as in RRSn=SnRR. A stannyne contains a carbon to tin triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
and a distannyne a triple bond between two tin atoms (RSnSnR) . Tin radicals are called stannyl radicals and tin carbenes stannylenes (RSn:)
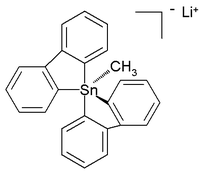
Hypercoordinated stannanes
Unlike their carbon analogues, tin compounds can also be coordinated to five and even six atoms instead of the regular four. These hypercoordinated compounds usually have electronegative substituents for stabilization. Lithium pentaorganostannates were first detected and characterized in solution in 1986, while in the subsequent year a six-coordinated tetraorganotin compound was reported. In 2007 a crystal structure of room-temperature stable (in argonArgon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
) all-carbon pentaorganostannane was reported as the lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
salt with this structure:
In this distorted trigonal bipyramidal structure
Trigonal bipyramid molecular geometry
In chemistry a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular dipyramid...
the carbon to tin bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s (2.26Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
apical, 2.17Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
equatorial) are larger than regular C-Sn bonds (2.14Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
) reflecting its hypervalent nature.