Stability constants of complexes
Encyclopedia
A stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex
. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host-guest complexes and complexes of anions. The stability constant(s) provide the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
had proposed the correct structures for coordination complexes, have been summarised by Beck and Nagypál. The key to Bjerrum’s method was the use of the then recently developed glass electrode
and pH meter
to determine the concentration of hydrogen ions in solution. Bjerrum recognised that the formation of a metal complex with a ligand was a kind of acid-base equilibrium: there is competition for the ligand, L, between the metal ion, Mn+, and the hydrogen ion, H+. This means that there are two simultaneous equilibria that have to be considered. In what follows electrical charges are omitted for the sake of generality. The two equilibria are
Hence by following the hydrogen ion concentration during a titration
of a mixture of M and HL with base
, and knowing the acid dissociation constant
of HL, the stability constant for the formation of ML could be determined. Bjerrum went on to determine the stability constants for systems in which many complexes may be formed.
The following twenty years saw a veritable explosion in the number of stability constants that were determined. Relationships, such as the Irving-Williams series
were discovered. The calculations were done by hand using the so-called graphical methods. The mathematics underlying the methods used in this period are summarised by Rossotti and Rossotti. The next key development was the use of a computer program, LETAGROP to do the calculations. This permitted the examination of systems too complicated to be evaluated by means of hand-calculations. Subsequently computer programs capable of handling complex equilibria in general, such as SCOGS and MINIQUAD were developed so that today the determination of stability constants has almost become a “routine” operation. Values of thousands of stability constants can be found in two commercial databases.
s, metal ions will be present as aqua-ions
, so the reaction for the formation of the first complex could be written as
The equilibrium constant for this reaction is given by
[L] should be read as "the concentration of L" and likewise for the other terms in square brackets. The expression can be greatly simplified by removing those terms which are constant. The number of water molecules attached to each metal ion is constant. In dilute solutions the concentration of water is effectively constant. The expression becomes
Following this simplification a general definition can be given, For the general equilibrium
The definition can easily be extended to include any number of reagents. The reagents need not always be a metal and a ligand but can be any species which form a complex. Stability constants defined in this way, are association constants. This can lead to some confusion as pKa values
are dissociation constants. In general purpose computer programs it is customary to define all constants as association constants. The relationship between the two types of constant is given in association and dissociation constants.

The stepwise constants, K1 and K2 refer to the formation of the complexes one step at a time.

It follows that
A cumulative constant can always be expressed as the product of stepwise constants. Conversely, any stepwise constant can be expressed as a quotient of two or more overall constants. There is no agreed notation for stepwise constants, though a symbol such as is sometimes found in the literature. It is best always to define each stability constant by reference to an equilibrium expression.
is sometimes found in the literature. It is best always to define each stability constant by reference to an equilibrium expression.
In water the concentration of hydroxide
is related to the concentration of hydrogen ions by the self-ionization constant
, Kw.
The expression for hydroxide concentration is substituted into the formation constant expression

The literature usually gives value of β*.
, A, and a Lewis base, B, can be considered to form a complex AB
There are three major theories relating to the strength of Lewis acids and bases and the interactions between them.
For more details see: acid-base reaction, acid catalysis
, acid-base extraction
and entropic
effects. Enthalpic effects depend on bond strengths and entropic effects have to do with changes in the order/disorder of the solution as a whole. The chelate effect, below, is best explained in terms of thermodynamics.
An equilibrium constant is related to the standard Gibbs free energy
change for the reaction
R is the gas constant
and T is the absolute temperature
. At 25 °C ΔG in kJ mol−1 = 5.708 log β (1 kJ mol−1 = 1000 Joule
in kJ mol−1 = 5.708 log β (1 kJ mol−1 = 1000 Joule
s per mole
). Free energy is made up of an enthalpy term and an entropy term. = ΔH
= ΔH − TΔS
− TΔS
The standard enthalpy change can be determined by calorimetry
or by using the van 't Hoff equation, though the calorimetric method is preferable. When both the standard enthalpy change and stability constant have been determined, the standard entropy change is easily calculated from the equation above.
The fact that stepwise formation constants of complexes of the type MLn decrease in magnitude as n increases may be partly explained in terms of the entropy factor. Take the case of the formation of octahedral complexes.
For the first step m=6, n=1 and the ligand can go into one of 6 sites. For the second step m=5 and the second ligand can go into one of only 5 sites. This means that there is more randomness in the first step than the second one; ΔS is more positive, so ΔG
is more positive, so ΔG is more negative and log K1 > log K2 . The ratio of the stepwise stability constants can be calculated on this basis, but experimental ratios are not exactly the same because ΔH
is more negative and log K1 > log K2 . The ratio of the stepwise stability constants can be calculated on this basis, but experimental ratios are not exactly the same because ΔH is not necessarily the same for each step. The entropy factor is also important in the chelate effect, below.
is not necessarily the same for each step. The entropy factor is also important in the chelate effect, below.
 , for the equilibrium
, for the equilibrium
can be defined as
where {ML} is the activity
of the chemical species ML etc. K is dimensionless since activity is dimensionless . Activities of the products are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient
is dimensionless since activity is dimensionless . Activities of the products are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient
for a derivation of this expression.
Since activity is the product of concentration
and activity coefficient
(γ) the definition could also be written as
where [ML] represents the concentration of ML and Γ is a quotient of activity coefficients. This expression can be generalized as
To avoid the complications involved in using activities, stability constants are determined
, where possible, in a medium consisting of a solution of a background electrolyte
at high ionic strength
, that is, under conditions in which Γ can be assumed to be always constant. For example, the medium might be a solution of 0.1 mol/dm−3 sodium nitrate
or 3 mol/dm−3 potassium perchlorate
. When Γ is constant it may be ignored and the general expression in theory, above, is obtained.
All published stability constant values refer to the specific ionic medium used in their determination and different values are obtained with different conditions, as illustrated for the complex CuL (L=glycinate). Furthermore, stability constant values depend on the specific electrolyte used as the value of Γ is different for different electrolytes, even at the same ionic strength. There does not need to be any chemical interaction between the species in equilibrium and the background electrolyte, but such interactions might occur in particular cases. For example, phosphates form weak complexes with alkali metals, so, when determining stability constants involving phosphates, such as ATP
, the background electrolyte used will be, for example, a tetralkylammonium
salt. Another example involves iron(III) which forms weak complexes with halide
and other anions, but not with perchlorate
ions.
When published constants refer to an ionic strength other than the one required for a particular application, they may be adjusted by means of specific ion theory (SIT) and other theories.
according to the van 't Hoff equation
R is the gas constant
and T is the thermodynamic temperature . Thus, for exothermic
reactions, (the standard enthalpy change, ΔH , is negative) K decreases with temperature, but for endothermic
, is negative) K decreases with temperature, but for endothermic
reactions (ΔH is positive) K increases with temperature.
is positive) K increases with temperature.
(II) ion, Cu2+ and ethylenediamine (en) on the one hand and methylamine
, MeNH2 on the other.
In (1) the bidentate ligand ethylene diamine forms a chelate complex with the copper ion. Chelation results in the formation of a five–membered ring. In (2) the bidentate ligand is replaced by two monodentate methylamine ligands of approximately the same donor power, meaning that the enthalpy
of formation of Cu—N bonds is approximately the same in the two reactions. Under conditions of equal copper concentrations and when then concentration of methylamine is twice the concentration of ethylenediamine, the concentration of the complex (1) will be greater than the concentration of the complex (2). The effect increases with the number of chelate rings so the concentration of the EDTA
complex, which has six chelate rings, is much higher than a corresponding complex with two monodentate nitrogen donor ligands and four monodentate carboxylate ligands. Thus, the phenomenon
of the chelate effect is a firmly established empirical
fact: under comparable conditions, the concentration of a chelate complex will be higher than the concentration of an analogous complex with monodentate ligands.
The thermodynamic
approach to explaining the chelate effect considers the equilibrium constant for the reaction: the larger the equilibrium constant, the higher the concentration of the complex.
When the analytical concentration of methylamine is twice that of ethylenediamine and the concentration of copper is the same in both reactions, the concentration [Cu(en)]2+ is much higher than the concentration [Cu(MeNH2)2]2+ because β11 >> β12.
The difference between the two stability constants is mainly due to the difference in the standard entropy change, ΔS . In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less entropy of disorder
. In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less entropy of disorder
is lost when the chelate complex is formed than when the complex with monodentate ligands is formed. This is one of the factors contributing to the entropy difference. Other factors include solvation changes and ring formation. Some experimental data to illustrate the effect are shown in the following table.
These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favourable in this instance. In general it is difficult to account precisely for thermodynamic values in terms of changes in solution at the molecular level, but it is clear that the chelate effect is predominantly an effect of entropy. Other explanations, Including that of Schwarzenbach, are discussed in Greenwood and Earnshaw.
The chelate effect increases as the number of chelate rings increases. For example the complex [Ni(dien)2)]2+ is more stable than the complex [Ni(en)3)]2+; both complexes are octahedral with six nitrogen atoms around the nickel ion, but dien (diethylenetriamine, 1,4,7-triazaheptane) is a tridentate
ligand and en is bidentate. The number of chelate rings is one less than the number of donor atoms in the ligand. EDTA
(ethylenediaminetetracetic acid) has six donor atoms so it forms very strong complexes with five chelate rings. Ligands such as DTPA, which have eight donor atoms are used to form complexes with large metal ions such as lanthanide
or actinide
ions which usually form 8- or 9- coordinate complexes.
5-membered and 6-membered chelate rings give the most stable complexes. 4-membered rings are subject to internal strain because of the small inter-bond angle is the ring. The chelate effect is also reduced with 7- and 8- membered rings, because the larger rings are less rigid, so less entropy is lost in forming them.
This phenomenon was named "the macrocyclic effect" and it was also interpreted as an entropy effect. However, later studies suggested that both enthalpy and entropy factors were involved.
An important difference between macrocyclic ligands and open-chain (chelating) ligands is that they have selectivity for metal ions, based on the size of the cavity into which the metal ion is inserted when a complex is formed. For example, the crown ether
18-crown-6 forms much stronger complexes with the potassium ion, K+ than with the smaller sodium ion, Na+.
In hemoglobin
an iron(II) ion is complexed by a macrocyclic porphyrin
ring. The article hemoglobin
incorrectly states that oxyhemoglogin contains iron(III). It is now known that the iron(II) in hemoglobin is a low-spin complex, whereas in oxyhemoglobin it is a high-spin complex. The low-spin Fe2+ ion fits snugly into the cavity of the porhyrin ring, but high-spin iron(II) is significantly larger and the iron atom is forced out of the plane of the macrocyclic ligand. This effect contributes the ability of hemoglobin to bind oxygen reversibly under biological conditions. In Vitamin B12
a cobalt(II) ion is held in a corrin ring. Chlorophyll
is a macrocyclic complex of magnesium(II).
In this case, K2 > K1. The reason for this is that, in aqueous solution, the ion written as Ag+ actually exists as the four-coordinate tetrahedral aqua species [Ag(OH2)4]+. The first step is then a substitution rreaction involving the displacement of a bound water molecule by ammonia forming the tetrahedral complex [Ag(NH3)(OH2)3]+ (commonly abbreviated as [Ag(NH3)]+). In the second step, the aqua ligands are lost to form a linear, two-coordinate product [H3N—Ag—NH3]+. Examination of the thermodynamic data shows that both enthalpy and entropy effects determine the result.
Other examples exist where the change is from octahedral to tetrahedral, as in the formation of [CoCl4]2− from [Co(H2O)6]2+.
s than with phosphines, but Pd2+ forms stronger complexes with phosphines than with amines. Later, Pearson proposed the theory of hard and soft acids and bases
(HSAB theory). In this classification, class A metals are hard acids and class B metals are soft acids. Some ions, such as copper(i) anr classed as borderline. Hard acids form stronger complexes with hard bases than with soft bases. In general terms hard-hard interactions are predominantly electrostatic in nature whereas soft-soft interactions are predominantly covalent in nature. The HSAB theory, though useful, is only semi-quantitative.
The hardness of a metal ion increases with oxidation state. An example of this effect is given by the fact that Fe2+ tends to form stronger complexes with N-donor ligands than with O-donor ligands, but the opposite is true for Fe3+.
refers to high-spin, octahedral, divalent metal ion of the first transition series. It places the stabilities of complexes in the order
This order was found to hold for a wide variety of ligands. There are three strands to the explanation of the series.
Another example of the effect of ionic radius the steady increase in stability of complexes with a given ligand along the series of trivalent lanthanide ions, an effect of the well-known lanthanide contraction
.
is used in the treatment of various metal-related illnesses, such as iron overload in β-thalassemia
sufferers who have been given blood transfusions. The ideal ligand binds to the target metal ion and not to others, but this degree of selectivity is very hard to achieve. The synthetic drug Deferiprone
achieves selectivity by having two oxygen donor atoms so that it binds to Fe3+ in preference to any of the other divalent ions that are present in the human body, such as Mg2+, Ca2+ and Zn2+. Treatment of poisoning by ions such as Pb2+ and Cd2+ is much more difficult since these are both divalent ions and selectivity is harder to accomplish. Excess copper in Wilson's disease
can be removed by penicillamine
or Triethylene tetramine
(TETA). DTPA has been approved by the U.S. Food and Drug Administration for treatment of plutonium
poisoning.
DTPA is also used as a complexing agent for gadolinium
in MRI contrast enhancement
. The requirement in this case is that the complex be very strong, as Gd3+ is very toxic. The large stability constant of the octadentate ligand ensures that the concentration of free Gd3+ is almost negligible, certainly well below toxicity threshold. In addition the ligand occupies only 8 of the 9 coordination sites on the gadolinium ion. The ninth site is occupied by a water molecule which exchanges rapidly with the fluid surrounding it and it is this mechanism that makes the paramagnetic complex into a contrast reagent.
EDTA
forms such strong complexes with most divalent cations that it finds many uses. For example, it is often present in washing powder to act as a water softener by sequestering calcium and magnesium ions.
The selectivity of macrocyclic ligands can be used as a basis for the construction of an ion selective electrode
. For example, potassium selective electrode
s are available that make use of the naturally-occurring macrocyclic antibiotic valinomycin
.
An ion-exchange resin such as chelex 100
, which contains chelating ligands bound to a polymer, can be used in water softeners and in chromatographic separation techniques. In solvent extraction the formation of electrically-neutral complexes allows cations to be extracted into organic solvents. For example, in nuclear fuel reprocessing
uranium(VI) and plutonium(VI) are extracted into kerosene as the complexes [MO2(TBP)2(NO3)2] (TBP = tri-'n-butyl phosphate). In phase-transfer catalysis, a substance which is insoluble in an organic solvent can be made soluble by addition of a suitable ligand. For example, potassium permanganate
oxidations can be achieved by adding a catalytic quantity of a crown ether and a small amount of organic solvent to the aqueous reaction mixture, so that the oxidation reaction occurs in the organic phase.
In all these examples, the ligand is chosen on the basis of the stability constants of the complexes formed. For example, TBP is used in nuclear fuel reprocessing because (among other reasons) it forms a complex strong enough for solvent extraction to take place, but weak enough that the complex can be destroyed by nitric acid to recover the uranyl cation as nitrato complexes, such as [UO2(NO3)4]2- back in the aqueous phase.
. Applications include molecular recognition
, host-guest chemistry
and anion sensors.
A typical application in molecular recognition involved the determination of formation constants for complexes formed between a tripodal substituted urea molecule and various saccharides. The study was carried out using a non-aqueous solvent and NMR chemical shift measurements. The object was to examine the selectivity with respect to the saccharides.
An example of the use of supramolecular complexes in the development of chemosensor
s is provided by the use of transition-metal ensembles to sense for ATP
.
Anion complexation can be achieved by encapsulating the anion in a suitable cage. Selectivity can be engineered by designing the shape of the cage. For example, dicarboxylate anions could be encapsulated in the ellipsoidal cavity in a large macrocyclic structure containing two metal ions.
is first acidified to the point where the ligand is fully protonated. This solution is then titrated, often by means of a computer-controlled auto-titrator, with a solution of CO2-free base. The concentration, or activity, of the hydrogen ion is monitored by means of a glass electrode. The data set used for the calculation has three components: a statement defining the nature of the chemical species that will be present, called the model of the system, details concerning the concentrations of the reagents used in the titration, and finally the experimental measurements in the form of titre and pH (or emf) pairs.
It is not always possible to use a glass electrode. If that is the case, the titration can be monitored by other types of measurement. Absorbance spectra, fluorescence spectra and NMR spectra are the most commonly used alternatives. Current practice is to take absorbance or fluorescence measurements at a range of wavelengths and to fit these data simultaneously. Various NMR chemical shifts can also be fitted together.
The chemical model will include values of the protonation constants of the ligand, which will have been determined in separate experiments, a value for log Kw and estimates of the unknown stability constants of the complexes formed. These estimates are necessary because the calculation uses a non-linear least-squares algorithm. The estimates are usually obtained by reference to a chemically similar system. The stability constant databases can be very useful in finding published stability constant values for related complexes.
In some simple cases the calculations can be done in a spreadsheet. Otherwise, the calculations are performed with the aid of a general-purpose computer programs. The most frequently used programs are:
In biochemistry, formation constants of adducts may be obtained from Isothermal titration calorimetry
(ITC) measurements. This technique yields both the stability constant and the standard enthalpy change for the equilibrium. It is mostly limited, by availability of software, to complexes of 1:1 stoichiometry.
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host-guest complexes and complexes of anions. The stability constant(s) provide the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
History
Jannik Bjerrum developed the first general method for the determination of stability constants of metal-ammine complexes in 1941. The reasons why this occurred at such a late date, nearly 50 years after Alfred WernerAlfred Werner
Alfred Werner was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of transition metal complexes. Werner developed the basis for modern coordination chemistry...
had proposed the correct structures for coordination complexes, have been summarised by Beck and Nagypál. The key to Bjerrum’s method was the use of the then recently developed glass electrode
Glass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. It is an important part of the instrumentation for chemical analysis and physico-chemical studies. In modern practice, widely used membranous ion-selective electrodes are part...
and pH meter
PH meter
A pH meter is an electronic instrument used for measuring the pH of a liquid...
to determine the concentration of hydrogen ions in solution. Bjerrum recognised that the formation of a metal complex with a ligand was a kind of acid-base equilibrium: there is competition for the ligand, L, between the metal ion, Mn+, and the hydrogen ion, H+. This means that there are two simultaneous equilibria that have to be considered. In what follows electrical charges are omitted for the sake of generality. The two equilibria are
- H + L HL
- M + L ML
Hence by following the hydrogen ion concentration during a titration
Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
of a mixture of M and HL with base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
, and knowing the acid dissociation constant
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of HL, the stability constant for the formation of ML could be determined. Bjerrum went on to determine the stability constants for systems in which many complexes may be formed.
- M + qL MLq
The following twenty years saw a veritable explosion in the number of stability constants that were determined. Relationships, such as the Irving-Williams series
Irving-Williams series
The Irving-Williams Series refers to the relative stabilities of complexes formed by a metal ion. For high-spin complexes of the divalent ions of first-row transition metals, the stability constant for the formation of a complex follows the orderThe Irving-Williams Series refers to the relative...
were discovered. The calculations were done by hand using the so-called graphical methods. The mathematics underlying the methods used in this period are summarised by Rossotti and Rossotti. The next key development was the use of a computer program, LETAGROP to do the calculations. This permitted the examination of systems too complicated to be evaluated by means of hand-calculations. Subsequently computer programs capable of handling complex equilibria in general, such as SCOGS and MINIQUAD were developed so that today the determination of stability constants has almost become a “routine” operation. Values of thousands of stability constants can be found in two commercial databases.
Theory
The formation of a complex between a metal ion, M, and a ligand, L, is in fact usually a substitution reaction. For example, In aqueous solutionAqueous solution
An aqueous solution is a solution in which the solvent is water. It is usually shown in chemical equations by appending aq to the relevant formula, such as NaCl. The word aqueous means pertaining to, related to, similar to, or dissolved in water...
s, metal ions will be present as aqua-ions
Metal ions in aqueous solution
A metal ion in aqueous solution is a cation, dissolved in water, of chemical formula [Mn]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for elements in rows 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have solvation...
, so the reaction for the formation of the first complex could be written as
- [M(H2O)n] + L [M(H2O)n-1L] +H2O
The equilibrium constant for this reaction is given by

[L] should be read as "the concentration of L" and likewise for the other terms in square brackets. The expression can be greatly simplified by removing those terms which are constant. The number of water molecules attached to each metal ion is constant. In dilute solutions the concentration of water is effectively constant. The expression becomes

Following this simplification a general definition can be given, For the general equilibrium
- pM + qL ... MpLq...

The definition can easily be extended to include any number of reagents. The reagents need not always be a metal and a ligand but can be any species which form a complex. Stability constants defined in this way, are association constants. This can lead to some confusion as pKa values
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
are dissociation constants. In general purpose computer programs it is customary to define all constants as association constants. The relationship between the two types of constant is given in association and dissociation constants.
Stepwise and cumulative constants
A cumulative or overall constant, given the symbol β, is the constant for the formation of a complex from reagents. For example, the cumulative constant for the formation of ML2 is given by
The stepwise constants, K1 and K2 refer to the formation of the complexes one step at a time.


It follows that

A cumulative constant can always be expressed as the product of stepwise constants. Conversely, any stepwise constant can be expressed as a quotient of two or more overall constants. There is no agreed notation for stepwise constants, though a symbol such as
 is sometimes found in the literature. It is best always to define each stability constant by reference to an equilibrium expression.
is sometimes found in the literature. It is best always to define each stability constant by reference to an equilibrium expression.Hydrolysis products
The formation of an hydroxo-complex is a typical example of an hydrolysis reaction. An hydrolysis reaction is one in which a substrate reacts with water, splitting a water molecule into hydroxide and hydrogen ions. In this case the hydroxide ion then forms a complex with the substrate.- M + OH M(OH)

In water the concentration of hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
is related to the concentration of hydrogen ions by the self-ionization constant
Self-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
, Kw.
- Kw=[H+][OH-]; [OH-] = Kw[H+]-1
The expression for hydroxide concentration is substituted into the formation constant expression


The literature usually gives value of β*.
Acid-base complexes
A Lewis acidLewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
, A, and a Lewis base, B, can be considered to form a complex AB

There are three major theories relating to the strength of Lewis acids and bases and the interactions between them.
- Hard and soft acid-base theory (HSAB). This is used mainly for qualitative purposes.
- Drago and Wayland proposed a two-parameter equation which predicts the standard enthalpy of formation of a very large number of adducts quite accurately. –ΔH
 (A—B) = EAEB + CACB. Values of the E and C parameters are available
(A—B) = EAEB + CACB. Values of the E and C parameters are available - Guttmann donor numberDonor numberIn chemistry a donor number or DN is a qualitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 , in dilute solution in the noncoordinating solvent 1,2-dichloroethane with a...
s: for bases the number is derived from the enthalpy of reaction of the base with antimony pentachlorideAntimony pentachlorideAntimony pentachloride is a the chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to impurities...
in 1,2-Dichloroethane1,2-DichloroethaneThe chemical compound 1,2-dichloroethane, commonly known by its old name of ethylene dichloride , is a chlorinated hydrocarbon, mainly used to produce vinyl chloride monomer , the major precursor for PVC production. It is a colourless liquid with a chloroform-like odour...
as solvent. For acids, an acceptor number is derived from the enthalpy of reaction of the acid with triphenylphosphine oxideTriphenylphosphine oxideTriphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
.
For more details see: acid-base reaction, acid catalysis
Acid catalysis
In acid catalysis and base catalysis a chemical reaction is catalyzed by an acid or a base. The acid is often the proton and the base is often a hydroxyl ion. Typical reactions catalyzed by proton transfer are esterfications and aldol reactions. In these reactions the conjugate acid of the carbonyl...
, acid-base extraction
Acid-base extraction
Acid-base extraction is a procedure using sequential liquid–liquid extractions to purify acids and bases from mixtures based on their chemical properties....
Thermodynamics
The thermodynamics of metal ion complex formation provides much significant information. In particular it is useful in distinguishing between enthalpicEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
and entropic
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
effects. Enthalpic effects depend on bond strengths and entropic effects have to do with changes in the order/disorder of the solution as a whole. The chelate effect, below, is best explained in terms of thermodynamics.
An equilibrium constant is related to the standard Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
change for the reaction
- ΔG
 = -2.303 RT log10 β.
= -2.303 RT log10 β.
R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and T is the absolute temperature
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
. At 25 °C ΔG
 in kJ mol−1 = 5.708 log β (1 kJ mol−1 = 1000 Joule
in kJ mol−1 = 5.708 log β (1 kJ mol−1 = 1000 JouleJoule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
s per mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
). Free energy is made up of an enthalpy term and an entropy term.
- Δ
 = ΔH
= ΔH − TΔS
− TΔS
The standard enthalpy change can be determined by calorimetry
Calorimetry
Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat...
or by using the van 't Hoff equation, though the calorimetric method is preferable. When both the standard enthalpy change and stability constant have been determined, the standard entropy change is easily calculated from the equation above.
The fact that stepwise formation constants of complexes of the type MLn decrease in magnitude as n increases may be partly explained in terms of the entropy factor. Take the case of the formation of octahedral complexes.
- [M(H2O)
For the first step m=6, n=1 and the ligand can go into one of 6 sites. For the second step m=5 and the second ligand can go into one of only 5 sites. This means that there is more randomness in the first step than the second one; ΔS
 is more positive, so ΔG
is more positive, so ΔG is more negative and log K1 > log K2 . The ratio of the stepwise stability constants can be calculated on this basis, but experimental ratios are not exactly the same because ΔH
is more negative and log K1 > log K2 . The ratio of the stepwise stability constants can be calculated on this basis, but experimental ratios are not exactly the same because ΔH is not necessarily the same for each step. The entropy factor is also important in the chelate effect, below.
is not necessarily the same for each step. The entropy factor is also important in the chelate effect, below.Ionic strength dependence
The thermodynamic equilibrium constant, K , for the equilibrium
, for the equilibrium
- M + L ML
can be defined as

where {ML} is the activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
of the chemical species ML etc. K
 is dimensionless since activity is dimensionless . Activities of the products are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient
is dimensionless since activity is dimensionless . Activities of the products are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficientActivity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
for a derivation of this expression.
Since activity is the product of concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
and activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
(γ) the definition could also be written as

where [ML] represents the concentration of ML and Γ is a quotient of activity coefficients. This expression can be generalized as

To avoid the complications involved in using activities, stability constants are determined
Determination of equilibrium constants
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant is expressed as a concentration quotient,K=\frac...
, where possible, in a medium consisting of a solution of a background electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
at high ionic strength
Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
, that is, under conditions in which Γ can be assumed to be always constant. For example, the medium might be a solution of 0.1 mol/dm−3 sodium nitrate
Sodium nitrate
Sodium nitrate is the chemical compound with the formula NaNO3. This salt, also known as Chile saltpeter or Peru saltpeter to distinguish it from ordinary saltpeter, potassium nitrate, is a white solid which is very soluble in water...
or 3 mol/dm−3 potassium perchlorate
Potassium perchlorate
Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer and potentially reacts with many organic substances...
. When Γ is constant it may be ignored and the general expression in theory, above, is obtained.
All published stability constant values refer to the specific ionic medium used in their determination and different values are obtained with different conditions, as illustrated for the complex CuL (L=glycinate). Furthermore, stability constant values depend on the specific electrolyte used as the value of Γ is different for different electrolytes, even at the same ionic strength. There does not need to be any chemical interaction between the species in equilibrium and the background electrolyte, but such interactions might occur in particular cases. For example, phosphates form weak complexes with alkali metals, so, when determining stability constants involving phosphates, such as ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, the background electrolyte used will be, for example, a tetralkylammonium
Quaternary ammonium cation
Quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure NR4+, R being an alkyl group or an aryl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged,...
salt. Another example involves iron(III) which forms weak complexes with halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
and other anions, but not with perchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
ions.
When published constants refer to an ionic strength other than the one required for a particular application, they may be adjusted by means of specific ion theory (SIT) and other theories.
Temperature dependence
All equilibrium constants vary with temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
according to the van 't Hoff equation
R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and T is the thermodynamic temperature . Thus, for exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
reactions, (the standard enthalpy change, ΔH
 , is negative) K decreases with temperature, but for endothermic
, is negative) K decreases with temperature, but for endothermicEndothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
reactions (ΔH
 is positive) K increases with temperature.
is positive) K increases with temperature.The chelate effect
Consider the two equilibria, in aqueous solution, between the copperCopper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
(II) ion, Cu2+ and ethylenediamine (en) on the one hand and methylamine
Methylamine
Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized...
, MeNH2 on the other.
- Cu2+ + en [Cu(en)]2+ (1)
- Cu2+ + 2 MeNH2 [Cu(MeNH2)2]2+ (2)
In (1) the bidentate ligand ethylene diamine forms a chelate complex with the copper ion. Chelation results in the formation of a five–membered ring. In (2) the bidentate ligand is replaced by two monodentate methylamine ligands of approximately the same donor power, meaning that the enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
of formation of Cu—N bonds is approximately the same in the two reactions. Under conditions of equal copper concentrations and when then concentration of methylamine is twice the concentration of ethylenediamine, the concentration of the complex (1) will be greater than the concentration of the complex (2). The effect increases with the number of chelate rings so the concentration of the EDTA
EDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
complex, which has six chelate rings, is much higher than a corresponding complex with two monodentate nitrogen donor ligands and four monodentate carboxylate ligands. Thus, the phenomenon
Phenomenon
A phenomenon , plural phenomena, is any observable occurrence. Phenomena are often, but not always, understood as 'appearances' or 'experiences'...
of the chelate effect is a firmly established empirical
Empirical
The word empirical denotes information gained by means of observation or experimentation. Empirical data are data produced by an experiment or observation....
fact: under comparable conditions, the concentration of a chelate complex will be higher than the concentration of an analogous complex with monodentate ligands.
The thermodynamic
Equilibrium thermodynamics
Equilibrium Thermodynamics is the systematic study of transformations of matter and energy in systems as they approach equilibrium. The word equilibrium implies a state of balance. Equilibrium thermodynamics, in origins, derives from analysis of the Carnot cycle. Here, typically a system, as...
approach to explaining the chelate effect considers the equilibrium constant for the reaction: the larger the equilibrium constant, the higher the concentration of the complex.
- [Cu(en] =β11[Cu][en]
- [Cu(MeNH2)2]= β12[Cu][MeNH2]2
When the analytical concentration of methylamine is twice that of ethylenediamine and the concentration of copper is the same in both reactions, the concentration [Cu(en)]2+ is much higher than the concentration [Cu(MeNH2)2]2+ because β11 >> β12.
The difference between the two stability constants is mainly due to the difference in the standard entropy change, ΔS
 . In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less entropy of disorder
. In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less entropy of disorderEntropy (order and disorder)
In thermodynamics, entropy is commonly associated with the amount of order, disorder, and/or chaos in a thermodynamic system. This stems from Rudolf Clausius' 1862 assertion that any thermodynamic processes always "admits to being reduced to the alteration in some way or another of the arrangement...
is lost when the chelate complex is formed than when the complex with monodentate ligands is formed. This is one of the factors contributing to the entropy difference. Other factors include solvation changes and ring formation. Some experimental data to illustrate the effect are shown in the following table.
| Equilibrium | log β | ΔG | ΔH /kJ mol−1 /kJ mol−1 | −TΔS /kJ mol−1 /kJ mol−1 |
|---|---|---|---|---|
| Cd2+ + 4 MeNH2 Cd(MeNH2)42+ | 6.55 | -37.4 | -57.3 | 19.9 |
| Cd2+ + 2 en Cd(en)22+ | 10.62 | -60.67 | -56.48 | |
These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favourable in this instance. In general it is difficult to account precisely for thermodynamic values in terms of changes in solution at the molecular level, but it is clear that the chelate effect is predominantly an effect of entropy. Other explanations, Including that of Schwarzenbach, are discussed in Greenwood and Earnshaw.
The chelate effect increases as the number of chelate rings increases. For example the complex [Ni(dien)2)]2+ is more stable than the complex [Ni(en)3)]2+; both complexes are octahedral with six nitrogen atoms around the nickel ion, but dien (diethylenetriamine, 1,4,7-triazaheptane) is a tridentate
Denticity
Denticity refers to the number of atoms in a single ligand that bind to a central atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate...
ligand and en is bidentate. The number of chelate rings is one less than the number of donor atoms in the ligand. EDTA
EDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
(ethylenediaminetetracetic acid) has six donor atoms so it forms very strong complexes with five chelate rings. Ligands such as DTPA, which have eight donor atoms are used to form complexes with large metal ions such as lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
or actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
ions which usually form 8- or 9- coordinate complexes.
5-membered and 6-membered chelate rings give the most stable complexes. 4-membered rings are subject to internal strain because of the small inter-bond angle is the ring. The chelate effect is also reduced with 7- and 8- membered rings, because the larger rings are less rigid, so less entropy is lost in forming them.
| Ethylenediamine (en) | Diethylenetriamine (dien) |
The macrocyclic effect
It was found that the stability of the complex of copper(II) with the macrocyclic ligand cyclam (1,4,8,11-tetraazacyclotetradecane) was much greater than expected in comparison to the stability of the complex with the corresponding open-chain amine.This phenomenon was named "the macrocyclic effect" and it was also interpreted as an entropy effect. However, later studies suggested that both enthalpy and entropy factors were involved.
An important difference between macrocyclic ligands and open-chain (chelating) ligands is that they have selectivity for metal ions, based on the size of the cavity into which the metal ion is inserted when a complex is formed. For example, the crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
18-crown-6 forms much stronger complexes with the potassium ion, K+ than with the smaller sodium ion, Na+.
In hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
an iron(II) ion is complexed by a macrocyclic porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
ring. The article hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
incorrectly states that oxyhemoglogin contains iron(III). It is now known that the iron(II) in hemoglobin is a low-spin complex, whereas in oxyhemoglobin it is a high-spin complex. The low-spin Fe2+ ion fits snugly into the cavity of the porhyrin ring, but high-spin iron(II) is significantly larger and the iron atom is forced out of the plane of the macrocyclic ligand. This effect contributes the ability of hemoglobin to bind oxygen reversibly under biological conditions. In Vitamin B12
Vitamin B12
Vitamin B12, vitamin B12 or vitamin B-12, also called cobalamin, is a water-soluble vitamin with a key role in the normal functioning of the brain and nervous system, and for the formation of blood. It is one of the eight B vitamins...
a cobalt(II) ion is held in a corrin ring. Chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
is a macrocyclic complex of magnesium(II).
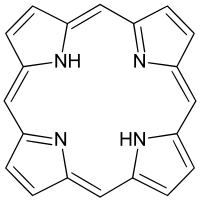 |
|
| Cyclam | Porphine, the simplest porphyrin. |
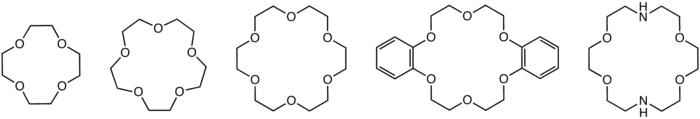 |
| Structures of common crown ethers: 12-crown-4 12-Crown-4 12-crown-4, also called 1,4,7,10-tetraoxacyclododecane and Lithium Ionophore V, is a crown ether with the formula C8H16O4. It is a cyclic tetramer of ethylene oxide which is specific for the lithium cation:-See also:*Crown ether... , 15-crown-5 15-Crown-5 15-Crown-5 is a crown ether with the formula C10H20O5. It is a cyclic pentamer of ethylene oxide that has been shown to complex with various cations, including sodium and potassium , but selective binding to lead .... , 18-crown-6 18-Crown-6 18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. The compound is a crown ether. Crown ethers coordinate some metal cations in their central cavity; 18-crown-6 displays a particular affinity for potassium cations. The synthesis... , dibenzo-18-crown-6 Dibenzo-18-crown-6 Dibenzo-18-crown-6 is a benzannulated crown ether. It is related to the non-benzannulated 18-crown-6. This compound may be synthesized from catechol and bisether:... , and diaza-18-crown-6 |
Geometrical factors
Successive stepwise formation constants Kn in a series such as MLn (n = 1, 2, ...) usually decrease as n increases. Exceptions to this rule occur when the geometry of the MLn complexes is not the same for all members of the series. The classic example is the formation of the diamminesilver(I) complex [Ag(NH3)2]+ in aqueous solution.- Ag+ + NH3 [Ag(NH3)]+

- Ag(NH3)+ + NH3 [Ag(NH3)2]+

In this case, K2 > K1. The reason for this is that, in aqueous solution, the ion written as Ag+ actually exists as the four-coordinate tetrahedral aqua species [Ag(OH2)4]+. The first step is then a substitution rreaction involving the displacement of a bound water molecule by ammonia forming the tetrahedral complex [Ag(NH3)(OH2)3]+ (commonly abbreviated as [Ag(NH3)]+). In the second step, the aqua ligands are lost to form a linear, two-coordinate product [H3N—Ag—NH3]+. Examination of the thermodynamic data shows that both enthalpy and entropy effects determine the result.
| equilibrium | ΔH /kJ mol−1 /kJ mol−1 |
ΔS /J K−1 mol−1 /J K−1 mol−1 |
|---|---|---|
| Ag+ + NH3 [Ag(NH3)]+ | −21.4 | 8.66 |
| [Ag(NH3)]+ + NH3 [Ag(NH3)2]+ | −35.2 | −61.26 |
Other examples exist where the change is from octahedral to tetrahedral, as in the formation of [CoCl4]2− from [Co(H2O)6]2+.
Classification of metal ions
Ahrland, Chatt and Davies proposed that metal ions could be described as class A if they formed stronger complexes with ligands whose donor atoms are N, O or F than with ligands whose donor atoms are P, S or Cl and class B if the reverse is true. For example, Ni2+ forms stronger complexes with amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s than with phosphines, but Pd2+ forms stronger complexes with phosphines than with amines. Later, Pearson proposed the theory of hard and soft acids and bases
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
(HSAB theory). In this classification, class A metals are hard acids and class B metals are soft acids. Some ions, such as copper(i) anr classed as borderline. Hard acids form stronger complexes with hard bases than with soft bases. In general terms hard-hard interactions are predominantly electrostatic in nature whereas soft-soft interactions are predominantly covalent in nature. The HSAB theory, though useful, is only semi-quantitative.
The hardness of a metal ion increases with oxidation state. An example of this effect is given by the fact that Fe2+ tends to form stronger complexes with N-donor ligands than with O-donor ligands, but the opposite is true for Fe3+.
Effect of ionic radius
The Irving-Williams seriesIrving-Williams series
The Irving-Williams Series refers to the relative stabilities of complexes formed by a metal ion. For high-spin complexes of the divalent ions of first-row transition metals, the stability constant for the formation of a complex follows the orderThe Irving-Williams Series refers to the relative...
refers to high-spin, octahedral, divalent metal ion of the first transition series. It places the stabilities of complexes in the order
- Mn < Fe < Co < Ni < Cu > Zn
This order was found to hold for a wide variety of ligands. There are three strands to the explanation of the series.
- The ionic radiusIonic radiusIonic radius, rion, is the radius of an atom's ion. Although neither atoms nor ions have sharp boundaries, it is important to treat them as if they are hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice...
is expected to decrease regularly for Mn2+ to Zn2+. This would be the normal periodic trend and would account for the general increase in stability. - The crystal field stabilisation energy (CFSE) increases from zero for manganese(II) to a maximum at nickel(II). This makes the complexes increasingly stable. CFSE returns to zero for zinc(II).
- Although the CFSE for copper(II) is less than for nickel(II), octahedral copper(II) complexes are subject to the Jahn-Teller effectJahn-Teller effectThe Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, or the Jahn–Teller theorem, describes the geometrical distortion of non-linear molecules under certain situations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory,...
which results in a complex having extra stability.
Another example of the effect of ionic radius the steady increase in stability of complexes with a given ligand along the series of trivalent lanthanide ions, an effect of the well-known lanthanide contraction
Lanthanide contraction
Lanthanide contraction is a term used in chemistry to describe the decrease in ionic radii of the elements in the lanthanide series from atomic number 58, Cerium to 71, Lutetium, which results in smaller than otherwise expected ionic radii for the subsequent elements starting with 72, Hafnium...
.
Applications
Stability constant values are exploited in a wide variety of applications. Chelation therapyChelation therapy
Chelation therapy is the administration of chelating agents to remove heavy metals from the body. For the most common forms of heavy metal intoxication—those involving lead, arsenic or mercury—the standard of care in the United States dictates the use of dimercaptosuccinic acid...
is used in the treatment of various metal-related illnesses, such as iron overload in β-thalassemia
Thalassemia
Thalassemia is an inherited autosomal recessive blood disease that originated in the Mediterranean region. In thalassemia the genetic defect, which could be either mutation or deletion, results in reduced rate of synthesis or no synthesis of one of the globin chains that make up hemoglobin...
sufferers who have been given blood transfusions. The ideal ligand binds to the target metal ion and not to others, but this degree of selectivity is very hard to achieve. The synthetic drug Deferiprone
Deferiprone
Deferiprone is an oral drug that chelates iron and is used to treat thalassaemia major.It has been licensed for use in Europe and Asia for many years while awaiting approval in Canada and the United States. On October 14, 2011, however, "the U.S...
achieves selectivity by having two oxygen donor atoms so that it binds to Fe3+ in preference to any of the other divalent ions that are present in the human body, such as Mg2+, Ca2+ and Zn2+. Treatment of poisoning by ions such as Pb2+ and Cd2+ is much more difficult since these are both divalent ions and selectivity is harder to accomplish. Excess copper in Wilson's disease
Wilson's disease
Wilson's disease or hepatolenticular degeneration is an autosomal recessive genetic disorder in which copper accumulates in tissues; this manifests as neurological or psychiatric symptoms and liver disease...
can be removed by penicillamine
Penicillamine
Penicillamine is a pharmaceutical of the chelator class. It is sold under the trade names of Cuprimine and Depen. The pharmaceutical form is D-penicillamine, as L-penicillamine is toxic...
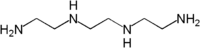
or Triethylene tetramine
Triethylene tetramine
Triethylenetetramine, abbreviated TETA and trien, is an organic compound with the formula [CH2NHCH2CH2NH2]2. This oily liquid is colourless but, like many amines, assumes a yellowish color due to impurities resulting from air-oxidation. It is soluble in polar solvents and exhibits the reactivity...
(TETA). DTPA has been approved by the U.S. Food and Drug Administration for treatment of plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
poisoning.
DTPA is also used as a complexing agent for gadolinium
Gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. It is a silvery-white, malleable and ductile rare-earth metal. It is found in nature only in combined form. Gadolinium was first detected spectroscopically in 1880 by de Marignac who separated its oxide and is credited with...
in MRI contrast enhancement
MRI contrast agent
MRI contrast agents are a group of contrast media used to improve the visibility of internal body structures in magnetic resonance imaging . The most commonly used compounds for contrast enhancement are gadolinium-based. MRI contrast agents alter the relaxation times of tissues and body cavities...
. The requirement in this case is that the complex be very strong, as Gd3+ is very toxic. The large stability constant of the octadentate ligand ensures that the concentration of free Gd3+ is almost negligible, certainly well below toxicity threshold. In addition the ligand occupies only 8 of the 9 coordination sites on the gadolinium ion. The ninth site is occupied by a water molecule which exchanges rapidly with the fluid surrounding it and it is this mechanism that makes the paramagnetic complex into a contrast reagent.
EDTA
EDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
forms such strong complexes with most divalent cations that it finds many uses. For example, it is often present in washing powder to act as a water softener by sequestering calcium and magnesium ions.
The selectivity of macrocyclic ligands can be used as a basis for the construction of an ion selective electrode
Ion selective electrode
An ion-selective electrode , also known as a specific ion electrode , is a transducer that converts the activity of a specific ion dissolved in a solution into an electrical potential, which can be measured by a voltmeter or pH meter. The voltage is theoretically dependent on the logarithm of the...
. For example, potassium selective electrode
Potassium selective electrode
Potassium selective electrodes are a type of ion selective electrode used in biochemical and biophysical research, where measurements of potassium concentration in an aqueous solution are required, usually on a real time basis....
s are available that make use of the naturally-occurring macrocyclic antibiotic valinomycin
Valinomycin
Valinomycin is a dodecadepsipeptide antibiotic.Valinomycin is obtained from the cells of several Streptomyces strains, among which "S. tsusimaensis" and S. fulvissimus....
.
 |
|||
| Deferiprone | penicillamine | triethylenetetramine, TETA | Ethylenediamine tetracetic acid, EDTA |
 |
 |
||
| diethylenetriaminepentacetic acid, DTPA | Valinomycin | tri-n-butylphosphate |
An ion-exchange resin such as chelex 100
Chelex 100
Chelex 100 is a chelating material from Bio-Rad used to purify other compounds via ion exchange. It is noteworthy for its ability to bind transition metal ions.It is a styrene-divinylbenzene co-polymer containing iminodiacetic acid groups....
, which contains chelating ligands bound to a polymer, can be used in water softeners and in chromatographic separation techniques. In solvent extraction the formation of electrically-neutral complexes allows cations to be extracted into organic solvents. For example, in nuclear fuel reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
uranium(VI) and plutonium(VI) are extracted into kerosene as the complexes [MO2(TBP)2(NO3)2] (TBP = tri-'n-butyl phosphate). In phase-transfer catalysis, a substance which is insoluble in an organic solvent can be made soluble by addition of a suitable ligand. For example, potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
oxidations can be achieved by adding a catalytic quantity of a crown ether and a small amount of organic solvent to the aqueous reaction mixture, so that the oxidation reaction occurs in the organic phase.
In all these examples, the ligand is chosen on the basis of the stability constants of the complexes formed. For example, TBP is used in nuclear fuel reprocessing because (among other reasons) it forms a complex strong enough for solvent extraction to take place, but weak enough that the complex can be destroyed by nitric acid to recover the uranyl cation as nitrato complexes, such as [UO2(NO3)4]2- back in the aqueous phase.
Supramolecular complexes
Supramolecular complexes are held together by hydrogen bonding, hydrophobic forces, van der Waals forces, π-π interactions, and electrostatic effects, all of which can be described as noncovalent bondingNoncovalent bonding
A noncovalent bond is a type of chemical bond, typically between macromolecules, that does not involve the sharing of pairs of electrons, but rather involves more dispersed variations of electromagnetic interactions. The noncovalent bond is the dominant type of bond between supermolecules in...
. Applications include molecular recognition
Molecular recognition
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, electrostatic and/or electromagnetic effects...
, host-guest chemistry
Host-guest chemistry
In supramolecular chemistry, host-guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host-guest chemistry encompasses the idea of molecular recognition...
and anion sensors.
A typical application in molecular recognition involved the determination of formation constants for complexes formed between a tripodal substituted urea molecule and various saccharides. The study was carried out using a non-aqueous solvent and NMR chemical shift measurements. The object was to examine the selectivity with respect to the saccharides.
An example of the use of supramolecular complexes in the development of chemosensor
Chemosensor
A chemoreceptor, also known as chemosensor, is a sensory receptor that transduces a chemical signal into an action potential. In more general terms, a chemosensor detects certain chemical stimuli in the environment.- Classes :...
s is provided by the use of transition-metal ensembles to sense for ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
.
Anion complexation can be achieved by encapsulating the anion in a suitable cage. Selectivity can be engineered by designing the shape of the cage. For example, dicarboxylate anions could be encapsulated in the ellipsoidal cavity in a large macrocyclic structure containing two metal ions.
Experimental methods
The method developed by Bjerrum is still the main method in use today, though the precision of the measurements has greatly increased. Most commonly, a solution containing the metal ion and the ligand in a medium of high ionic strengthIonic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
is first acidified to the point where the ligand is fully protonated. This solution is then titrated, often by means of a computer-controlled auto-titrator, with a solution of CO2-free base. The concentration, or activity, of the hydrogen ion is monitored by means of a glass electrode. The data set used for the calculation has three components: a statement defining the nature of the chemical species that will be present, called the model of the system, details concerning the concentrations of the reagents used in the titration, and finally the experimental measurements in the form of titre and pH (or emf) pairs.
It is not always possible to use a glass electrode. If that is the case, the titration can be monitored by other types of measurement. Absorbance spectra, fluorescence spectra and NMR spectra are the most commonly used alternatives. Current practice is to take absorbance or fluorescence measurements at a range of wavelengths and to fit these data simultaneously. Various NMR chemical shifts can also be fitted together.
The chemical model will include values of the protonation constants of the ligand, which will have been determined in separate experiments, a value for log Kw and estimates of the unknown stability constants of the complexes formed. These estimates are necessary because the calculation uses a non-linear least-squares algorithm. The estimates are usually obtained by reference to a chemically similar system. The stability constant databases can be very useful in finding published stability constant values for related complexes.
In some simple cases the calculations can be done in a spreadsheet. Otherwise, the calculations are performed with the aid of a general-purpose computer programs. The most frequently used programs are:
- Potentiometric and/or spectrophotometric data: HYPERQUAD, PSEQUAD
- Potentiometric data: BEST
- Spectrophotometric data: SQUAD, SPECFIT,
- NMR data HypNMR, EQNMR
In biochemistry, formation constants of adducts may be obtained from Isothermal titration calorimetry
Isothermal Titration Calorimetry
Isothermal titration calorimetry is a physical technique used to determine the thermodynamic parameters of interactions in solution. It is most often used to study the binding of small molecules to larger macromolecules .-Thermodynamic measurements:ITC is a quantitative technique that can...
(ITC) measurements. This technique yields both the stability constant and the standard enthalpy change for the equilibrium. It is mostly limited, by availability of software, to complexes of 1:1 stoichiometry.
Critically evaluated data
The following references are for critical reviews of published stability constants for various classes of ligands. All these reviews are published by IUPAC and the full text is available, free of charge, in pdf format.- ethylenediamine (en)
- Nitrilotriacetic acidNitrilotriacetic acidNitrilotriacetic acid , C6H9NO6, is a polyamino carboxylic acid and is used as a chelating agent which forms coordination compounds with metal ions such as Ca2+, Cu2+ or Fe3+.The uses of NTA are similar to that of EDTA...
(NTA)
- polyamino carboxylic acidPolyamino carboxylic acidthumb|left|120px|a metal complex with the [[EDTA]] anionthumb|120px|the [[glycine|glycinate]] ion can form a chelate complex with a metal ionthumb|120px| β [[alanine]]...
s (complexones)
- Alpha hydroxy acidAlpha hydroxy acidα-Hydroxy acids, or alpha hydroxy acids , are a class of chemical compounds that consist of a carboxylic acid substituted with a hydroxyl group on the adjacent carbon. They may be either naturally occurring or synthetic. AHAs are well-known for their use in the cosmetics industry...
s and other hydroxycarboxylic acids
- crown etherCrown etherCrown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
s
- phosphonic acids
- imidazoleImidazoleImidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
s and histamineHistamineHistamine is an organic nitrogen compound involved in local immune responses as well as regulating physiological function in the gut and acting as a neurotransmitter. Histamine triggers the inflammatory response. As part of an immune response to foreign pathogens, histamine is produced by...
s
- amino acidAmino acidAmino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s with polar side-chains
- nucleotides
- acetylacetoneAcetylacetoneAcetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
- general
- Chemical speciation of environmentally significant heavy metals with inorganic ligands. Part 1: The Hg2+– Cl–, OH–, CO32–, SO42–, and PO43– systems.
- Chemical speciation of environmentally significant metals with inorganic ligands Part 2: The Cu2+-OH-, Cl-, CO32-, SO42-, and PO43- aqueous systems
- Chemical speciation of environmentally significant metals with inorganic ligands Part 3: The Pb2+-OH-, Cl-, CO32-, SO42-, and PO43- systems
External links
- Stability constants web-site Contains information on computer programs, applications, databases and hardware for experimental titrations.

