
Ionic strength
Encyclopedia
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ion
ic compound
s, when dissolved in water, dissociate into ions. The total electrolyte
concentration in solution will affect important properties such as the dissociation or the solubility of different salt
s. One of the main characteristics of a solution with dissolved ions is the ionic strength.
of all ion
s present in that solution
.
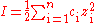
where ci is the molar concentration of ion i(mol·dm-3), zi is the charge
number of that ion, and the sum is taken over all ions in the solution. For a 1:1 electrolyte
such as sodium chloride
, the ionic strength is equal to the concentration, but for MgSO4
the ionic strength is four times higher. Generally multivalent ions contribute strongly to the ionic strength.
For example the ionic strength of a mixed 0.050 mol dm-3 in Na2SO4 and 0.020 mol dm-3 in NaCl solution is:
Another example is MgSO4
. The Magnesium cation has a 2 + charge, so the Mg 2+ contribution is .37M*2^2 and the SO4 2- contribution is .37M*2^2 . now to sum it up (.37*2^2)+(.37*2^2) is 2.96, but we still have to take one half of it, so 2.96*(1/2) is 1.48
____________________________________________________________________________________________________________________________________
Because in non-ideal solutions volumes are no longer strictly additive it is often preferable to work with molality (mol/kg{H2O}) rather than molarity (mol/L). In that case, ionic strength is defined as:
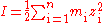
i = individual element
z = charge of element
that describes the strong deviations from ideality typically encountered in ionic solutions. It is also important for the theory of double layer
and related electrokinetic phenomena
and electroacoustic phenomena
in colloids
and other heterogeneous systems. That is, the Debye length, which is the inverse of the Debye
parameter (κ), is inversely proportional to the square root of the ionic strength. Debye length
is characteristic of the Double layer thickness. Increasing the concentration or valence
of the counterions compresses the double layer and increases the electrical potential gradient.
Media of high ionic strength are used in stability constant determination
in order to minimize changes, during a titration, in the activity quotient of solutes at lower concentrations. Natural waters such as seawater have a non-zero ionic strength due to the presence of dissolved salts which significantly affects their properties.
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
ic compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s, when dissolved in water, dissociate into ions. The total electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
concentration in solution will affect important properties such as the dissociation or the solubility of different salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
s. One of the main characteristics of a solution with dissolved ions is the ionic strength.
Quantifying ionic strength
The ionic strength, I, of a solution is a function of the concentrationConcentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of all ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s present in that solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
.
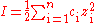
where ci is the molar concentration of ion i(mol·dm-3), zi is the charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
number of that ion, and the sum is taken over all ions in the solution. For a 1:1 electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
such as sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
, the ionic strength is equal to the concentration, but for MgSO4
Magnesium sulfate
Magnesium sulfate is a chemical compound containing magnesium, sulfur and oxygen, with the formula MgSO4. It is often encountered as the heptahydrate epsomite , commonly called Epsom salt, from the town of Epsom in Surrey, England, where the salt was distilled from the springs that arise where the...
the ionic strength is four times higher. Generally multivalent ions contribute strongly to the ionic strength.
For example the ionic strength of a mixed 0.050 mol dm-3 in Na2SO4 and 0.020 mol dm-3 in NaCl solution is:
I = 1/2((2 × (+1)2 × 0.050) + (+1)2 × 0.020 + (−2)2 × 0.050 + (−1)2 × 0.020) = 0.17 mol·dm-3
Another example is MgSO4
Magnesium sulfate
Magnesium sulfate is a chemical compound containing magnesium, sulfur and oxygen, with the formula MgSO4. It is often encountered as the heptahydrate epsomite , commonly called Epsom salt, from the town of Epsom in Surrey, England, where the salt was distilled from the springs that arise where the...
. The Magnesium cation has a 2 + charge, so the Mg 2+ contribution is .37M*2^2 and the SO4 2- contribution is .37M*2^2 . now to sum it up (.37*2^2)+(.37*2^2) is 2.96, but we still have to take one half of it, so 2.96*(1/2) is 1.48
____________________________________________________________________________________________________________________________________
Because in non-ideal solutions volumes are no longer strictly additive it is often preferable to work with molality (mol/kg{H2O}) rather than molarity (mol/L). In that case, ionic strength is defined as:
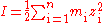
i = individual element
z = charge of element
Importance
The ionic strength plays a central role in the Debye–Hückel theoryDebye-Hückel equation
The Debye–Hückel equation and Debye–Hückel limiting law, were derived by Peter Debye and Erich Hückel, who developed a theory with which to calculate activity coefficients of electrolyte solutions. Activities, rather than concentrations, are needed in many chemical calculations because solutions...
that describes the strong deviations from ideality typically encountered in ionic solutions. It is also important for the theory of double layer
Double layer (interfacial)
A double layer is a structure that appears on the surface of an object when it is placed into a liquid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object...
and related electrokinetic phenomena
Electrokinetic phenomena
Electrokinetic phenomena are a family of several different effects that occur in heterogeneous fluids or in porous bodies filled with fluid. The term heterogeneous here means a fluid containing particles...
and electroacoustic phenomena
Electroacoustic phenomena
Electroacoustic phenomena arise when ultrasound propagates through a fluid containing ions. The associated particle motion generates electric signals because ions have electric charge. This coupling between ultrasound and electric field is called electroacoustic phenomena. Fluid might be a simple...
in colloids
DLVO theory
The DLVO theory is named after Derjaguin and Landau, Verwey and Overbeek.The theory describes the force between charged surfaces interacting through a liquid medium....
and other heterogeneous systems. That is, the Debye length, which is the inverse of the Debye
Peter Debye
Peter Joseph William Debye FRS was a Dutch physicist and physical chemist, and Nobel laureate in Chemistry.-Early life:...
parameter (κ), is inversely proportional to the square root of the ionic strength. Debye length
Debye length
In plasma physics, the Debye length , named after the Dutch physicist and physical chemist Peter Debye, is the scale over which mobile charge carriers screen out electric fields in plasmas and other conductors. In other words, the Debye length is the distance over which significant charge...
is characteristic of the Double layer thickness. Increasing the concentration or valence
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
of the counterions compresses the double layer and increases the electrical potential gradient.
Media of high ionic strength are used in stability constant determination
Determination of equilibrium constants
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant is expressed as a concentration quotient,K=\frac...
in order to minimize changes, during a titration, in the activity quotient of solutes at lower concentrations. Natural waters such as seawater have a non-zero ionic strength due to the presence of dissolved salts which significantly affects their properties.
See also
- Activity (chemistry)Activity (chemistry)In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
- Activity coefficientActivity coefficientAn activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
- Double layer (interfacial)Double layer (interfacial)A double layer is a structure that appears on the surface of an object when it is placed into a liquid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object...
- Double layer (electrode)
- Electrical double layer

