
Entropy (order and disorder)
Encyclopedia
In thermodynamics, entropy
is commonly associated with the amount of order, disorder, and/or chaos
in a thermodynamic system
. This stems from Rudolf Clausius
' 1862 assertion that any thermodynamic processes always "admits to being reduced to the alteration in some way or another of the arrangement of the constituent parts of the working body" and that internal work associated with these alterations is quantified energetically by a measure of "entropy" change, according to the following differential expression:
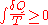
In the years to follow, Ludwig Boltzmann
translated these "alterations" into that of a probabilistic view of order and disorder in gas phase molecular systems.
In recent years, in chemistry textbooks there has been a shift away from using the terms "order" and "disorder" to that of the concept of energy dispersion
to describe entropy, among other theories. In the 2002 encyclopedia Encarta
, for example, entropy is defined as a thermodynamic property which serves as a measure of how close a system is to equilibrium; as well as a measure of the disorder in the system.
In the context of entropy, "perfect internal disorder" is synonymous with "equilibrium", but since that definition is so far different from the usual definition implied in normal speech, the use of the term in science has caused a great deal of confusion and misunderstanding.
Locally, the entropy can be lowered by external action. This applies to machines, such as a refrigerator, where the entropy in the cold chamber is being reduced, and to living organisms. This local decrease in entropy is, however, only possible at the expense of an entropy increase in the surroundings.
in the 1850s, particularly with his 1862 visual conception of molecular disgregation
. Similarly, in 1859, after reading a paper on the diffusion of molecules by Clausius, Scottish physicist James Clerk Maxwell
formulated the Maxwell distribution of molecular velocities, which gave the proportion of molecules having a certain velocity in a specific range. This was the first-ever statistical law in physics.
In 1864, Ludwig Boltzmann
, a young student in Vienna, came across Maxwell’s paper and was so inspired by it that he spent much of his long and distinguished life developing the subject further. Later, Boltzmann, in efforts to develop a kinetic theory
for the behavior of a gas, applied the laws of probability
to Maxwell's and Clausius' molecular interpretation of entropy so to begin to interpret entropy in terms of order and disorder. Similarly, in 1882 Hermann von Helmholtz
used the word "Unordnung" (disorder) to describe entropy.
Entropy and disorder also have associations with equilibrium
. Technically, entropy, from this perspective, is defined as a thermodynamic property which serves as a measure of how close a system is to equilibrium — that is, to perfect internal disorder. Likewise, the value of the entropy of a distribution of atoms and molecules in a thermodynamic system
is a measure of the disorder in the arrangements of its particles. In a stretched out piece of rubber, for example, the arrangement of the molecules of its structure has an “ordered” distribution and has zero entropy, while the “disordered” kinky distribution of the atoms and molecules in the rubber in the non-stretched state has positive entropy. Similarly, in a gas, the order is perfect and the measure of entropy of the system has its lowest value when all the molecules are in one place, whereas when more points are occupied the gas is all the more disorderly and the measure of the entropy of the system has its largest value.
In systems ecology
, as another example, the entropy of a collection of items comprising a system is defined as a measure of their disorder or equivalently the relative likelihood of the instantaneous configuration of the items. Moreover, according to theoretical ecologist and chemical engineer Robert Ulanowicz
, “that entropy might provide a quantification of the heretofore subjective notion of disorder has spawned innumerable scientific and philosophical narratives.” In particular, many biologists have taken to speaking in terms of the entropy of an organism, or about its antonym negentropy
, as a measure of the structural order within an organism.
The mathematical basis with respect to the association entropy has with order and disorder began, essentially, with the famous Boltzmann formula, S = k ln W, which relates entropy S to the number of possible states W in which a system can be found. As an example, consider a box that is divided into two sections. What is the probability that a certain number, or all of the particles, will be found in one section versus the other when the particles are randomly allocated to different places within the box? If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. If you have more than one particle, or define states as being further locational subdivisions of the box, the entropy is lower because the number of states is greater. The relationship between entropy, order, and disorder in the Boltzmann equation is so clear among physicists that according to the views of thermodynamic ecologists Sven Jorgensen and Yuri Svirezhev, “it is obvious that entropy is a measure of order or, most likely, disorder in the system.” In this direction, the second law of thermodynamics, as famously enunciated by Rudolf Clausius
in 1865, states that:
Thus, if entropy is associated with disorder and if the entropy of the universe is headed towards maximal entropy, then many are often puzzled as to the nature of the "ordering" process and operation of evolution
in relation to Clausius' most famous version of the second law, which states that the universe is headed towards maximal “disorder”. In the recent 2003 book SYNC – the Emerging Science of Spontaneous Order by Steven Strogatz
, for example, we find “Scientists have often been baffled by the existence of spontaneous order in the universe. The laws of thermodynamics
seem to dictate the opposite, that nature should inexorably degenerate toward a state of greater disorder, greater entropy. Yet all around us we see magnificent structures—galaxies, cells, ecosystems, human beings—that have all somehow managed to assemble themselves.”
The common argument used to explain this is that, locally, entropy can be lowered by external action, e.g. solar heating action, and that this applies to machines, such as a refrigerator, where the entropy in the cold chamber is being reduced, to growing crystals, and to living organisms. This local increase in order is, however, only possible at the expense of an entropy increase in the surroundings; here more disorder must be created. The conditioner of this statement suffices that living systems are open system
s in which both heat
, mass
, and or work
may transfer into or out of the system. Unlike temperature, the putative entropy of a living system would drastically change if the organism were thermodynamically isolated. If an organism was in this type of “isolated” situation, its entropy would increase markedly as the once-living components of the organism decayed to an unrecognizable mass.
, at absolute zero
temperature, crystalline structures are approximated to have perfect "order" and zero entropy. This correlation occurs because the numbers of different microscopic quantum energy states available to an ordered system are usually much smaller than the number of states available to a system that appears to be disordered.
From his famous 1896 Lectures on Gas Theory, Boltzmann diagrams the structure of a solid body, as shown above, by postulating that each molecule
in the body has a "rest position". According to Boltzmann, if it approaches a neighbor molecule it is repelled by it, but if it moves farther away there is an attraction. This, of course was a revolutionary perspective in its time; many, during these years, did not believe in the existence of either atoms or molecules (see: history of the molecule
). According to these early views, and others such as those developed by William Thomson
, if energy in the form of heat
is added to a solid, so to make it into a liquid or a gas, a common depiction is that the ordering of the atoms and molecules becomes more random and chaotic with an increase in temperature:
Thus, according to Boltzmann, owing to increases in thermal motion, whenever heat is added to a working substance, the rest position of molecules will be pushed apart, the body will expand, and this will create more molar-disordered distributions and arrangements of molecules. These disordered arrangements, subsequently, correlate, via probability arguments, to an increase in the measure of entropy.
being applied to the container using a powerful external magnet, so that the tiny molecular magnets are aligned forming a well-ordered "initial" state at that low temperature. This magnetic alignment means that the magnetic energy of each molecule is minimal. The external magnetic field is then reduced, a removal that is considered to be closely reversible. Following this reduction, the atomic magnets then assume random less-ordered orientations, owing to thermal agitations, in the "final" state:
The "disorder" and hence the entropy associated with the change in the atomic alignments has clearly increased. In terms of energy flow, the movement from a magnetically aligned state requires energy from the thermal motion of the molecules, converting thermal energy into magnetic energy. Yet, according to the second law of thermodynamics
, because no heat
can enter or leave the container, due to its adiabatic insulation, the system should exhibit no change in entropy, i.e. ΔS = 0. The increase in disorder, however, associated with the randomizing directions of the atomic magnets represents an entropy increase? To compensate for this, the disorder (entropy) associated with the temperature
of the specimen must decrease by the same amount. The temperature thus falls as a result of this process of thermal energy being converted into magnetic energy. If the magnetic field is then increased, the temperature rises and the magnetic salt has to be cooled again using a cold material such as liquid helium.
Whilst when considered at a microscopic level the term disorder may quite correctly suggest an increased range of accessible possibilities, confusion may be caused because at the macroscopic level of everyday perception a higher entropy state may appear more homogenous, more even or more smoothly mixed—apparently in diametric opposition to its description as being "more disordered". Thus for example there may be dissonance at equilibrium
being equated with "perfect internal disorder"; or the mixing of milk in coffee from apparent chaos to uniformity being described as a transition from an ordered state into a disordered state.
It has to be stressed, therefore, that "disorder", as used in a thermodynamic sense, relates to a full microscopic description of the system, rather than its apparent macroscopic properties. Many popular chemistry textbooks in recent editions increasingly have tended to instead present entropy through the idea of energy dispersal
, which is a dominant contribution to entropy in most everyday situations.
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
is commonly associated with the amount of order, disorder, and/or chaos
Chaos theory
Chaos theory is a field of study in mathematics, with applications in several disciplines including physics, economics, biology, and philosophy. Chaos theory studies the behavior of dynamical systems that are highly sensitive to initial conditions, an effect which is popularly referred to as the...
in a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
. This stems from Rudolf Clausius
Rudolf Clausius
Rudolf Julius Emanuel Clausius , was a German physicist and mathematician and is considered one of the central founders of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he put the theory of heat on a truer and sounder basis...
' 1862 assertion that any thermodynamic processes always "admits to being reduced to the alteration in some way or another of the arrangement of the constituent parts of the working body" and that internal work associated with these alterations is quantified energetically by a measure of "entropy" change, according to the following differential expression:
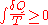
In the years to follow, Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
translated these "alterations" into that of a probabilistic view of order and disorder in gas phase molecular systems.
In recent years, in chemistry textbooks there has been a shift away from using the terms "order" and "disorder" to that of the concept of energy dispersion
Entropy (energy dispersal)
The description of entropy as energy dispersal provides an introductory method of teaching the thermodynamic concept of entropy. In physics and physical chemistry, entropy has commonly been defined as a scalar measure of the disorder of a thermodynamic system...
to describe entropy, among other theories. In the 2002 encyclopedia Encarta
Encarta
Microsoft Encarta was a digital multimedia encyclopedia published by Microsoft Corporation from 1993 to 2009. , the complete English version, Encarta Premium, consisted of more than 62,000 articles, numerous photos and illustrations, music clips, videos, interactive contents, timelines, maps and...
, for example, entropy is defined as a thermodynamic property which serves as a measure of how close a system is to equilibrium; as well as a measure of the disorder in the system.
In the context of entropy, "perfect internal disorder" is synonymous with "equilibrium", but since that definition is so far different from the usual definition implied in normal speech, the use of the term in science has caused a great deal of confusion and misunderstanding.
Locally, the entropy can be lowered by external action. This applies to machines, such as a refrigerator, where the entropy in the cold chamber is being reduced, and to living organisms. This local decrease in entropy is, however, only possible at the expense of an entropy increase in the surroundings.
History
This "molecular ordering" entropy perspective traces its origins to molecular movement interpretations developed by Rudolf ClausiusRudolf Clausius
Rudolf Julius Emanuel Clausius , was a German physicist and mathematician and is considered one of the central founders of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he put the theory of heat on a truer and sounder basis...
in the 1850s, particularly with his 1862 visual conception of molecular disgregation
Disgregation
In the history of thermodynamics, disgregation was defined in 1862 by Rudolf Clausius as the magnitude of the degree in which the molecules of a body are separated from each other...
. Similarly, in 1859, after reading a paper on the diffusion of molecules by Clausius, Scottish physicist James Clerk Maxwell
James Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
formulated the Maxwell distribution of molecular velocities, which gave the proportion of molecules having a certain velocity in a specific range. This was the first-ever statistical law in physics.
In 1864, Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
, a young student in Vienna, came across Maxwell’s paper and was so inspired by it that he spent much of his long and distinguished life developing the subject further. Later, Boltzmann, in efforts to develop a kinetic theory
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
for the behavior of a gas, applied the laws of probability
Probability
Probability is ordinarily used to describe an attitude of mind towards some proposition of whose truth we arenot certain. The proposition of interest is usually of the form "Will a specific event occur?" The attitude of mind is of the form "How certain are we that the event will occur?" The...
to Maxwell's and Clausius' molecular interpretation of entropy so to begin to interpret entropy in terms of order and disorder. Similarly, in 1882 Hermann von Helmholtz
Hermann von Helmholtz
Hermann Ludwig Ferdinand von Helmholtz was a German physician and physicist who made significant contributions to several widely varied areas of modern science...
used the word "Unordnung" (disorder) to describe entropy.
Overview
To highlight the fact that order and disorder are commonly understood to be measured in terms of entropy, below are current science encyclopedia and science dictionary definitions of entropy:- Entropy – a measure of the unavailability of a system’s energy to do work; also a measure of disorder; the higher the entropy the greater the disorder.
- Entropy – a measure of disorder; the higher the entropy the greater the disorder.
- Entropy – in thermodynamics, a parameter representing the state of disorder of a system at the atomic, ionic, or molecular level; the greater the disorder the higher the entropy.
- Entropy – a measure of disorder in the universe or of the availability of the energy in a system to do work.
Entropy and disorder also have associations with equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
. Technically, entropy, from this perspective, is defined as a thermodynamic property which serves as a measure of how close a system is to equilibrium — that is, to perfect internal disorder. Likewise, the value of the entropy of a distribution of atoms and molecules in a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
is a measure of the disorder in the arrangements of its particles. In a stretched out piece of rubber, for example, the arrangement of the molecules of its structure has an “ordered” distribution and has zero entropy, while the “disordered” kinky distribution of the atoms and molecules in the rubber in the non-stretched state has positive entropy. Similarly, in a gas, the order is perfect and the measure of entropy of the system has its lowest value when all the molecules are in one place, whereas when more points are occupied the gas is all the more disorderly and the measure of the entropy of the system has its largest value.
In systems ecology
Systems ecology
Systems ecology is an interdisciplinary field of ecology, taking a holistic approach to the study of ecological systems, especially ecosystems. Systems ecology can be seen as an application of general systems theory to ecology. Central to the systems ecology approach is the idea that an ecosystem...
, as another example, the entropy of a collection of items comprising a system is defined as a measure of their disorder or equivalently the relative likelihood of the instantaneous configuration of the items. Moreover, according to theoretical ecologist and chemical engineer Robert Ulanowicz
Robert Ulanowicz
Robert Edward Ulanowicz is an American theoretical ecologist and philosopher who is best known for his search for a unified theory of ecology. He was born September 17, 1943 in Baltimore, Maryland....
, “that entropy might provide a quantification of the heretofore subjective notion of disorder has spawned innumerable scientific and philosophical narratives.” In particular, many biologists have taken to speaking in terms of the entropy of an organism, or about its antonym negentropy
Negentropy
The negentropy, also negative entropy or syntropy, of a living system is the entropy that it exports to keep its own entropy low; it lies at the intersection of entropy and life...
, as a measure of the structural order within an organism.
The mathematical basis with respect to the association entropy has with order and disorder began, essentially, with the famous Boltzmann formula, S = k ln W, which relates entropy S to the number of possible states W in which a system can be found. As an example, consider a box that is divided into two sections. What is the probability that a certain number, or all of the particles, will be found in one section versus the other when the particles are randomly allocated to different places within the box? If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. If you have more than one particle, or define states as being further locational subdivisions of the box, the entropy is lower because the number of states is greater. The relationship between entropy, order, and disorder in the Boltzmann equation is so clear among physicists that according to the views of thermodynamic ecologists Sven Jorgensen and Yuri Svirezhev, “it is obvious that entropy is a measure of order or, most likely, disorder in the system.” In this direction, the second law of thermodynamics, as famously enunciated by Rudolf Clausius
Rudolf Clausius
Rudolf Julius Emanuel Clausius , was a German physicist and mathematician and is considered one of the central founders of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he put the theory of heat on a truer and sounder basis...
in 1865, states that:
Thus, if entropy is associated with disorder and if the entropy of the universe is headed towards maximal entropy, then many are often puzzled as to the nature of the "ordering" process and operation of evolution
Evolution
Evolution is any change across successive generations in the heritable characteristics of biological populations. Evolutionary processes give rise to diversity at every level of biological organisation, including species, individual organisms and molecules such as DNA and proteins.Life on Earth...
in relation to Clausius' most famous version of the second law, which states that the universe is headed towards maximal “disorder”. In the recent 2003 book SYNC – the Emerging Science of Spontaneous Order by Steven Strogatz
Steven Strogatz
Steven Henry Strogatz is an American mathematician and the Jacob Gould Schurman Professor of Applied Mathematics at Cornell University...
, for example, we find “Scientists have often been baffled by the existence of spontaneous order in the universe. The laws of thermodynamics
Laws of thermodynamics
The four laws of thermodynamics summarize its most important facts. They define fundamental physical quantities, such as temperature, energy, and entropy, in order to describe thermodynamic systems. They also describe the transfer of energy as heat and work in thermodynamic processes...
seem to dictate the opposite, that nature should inexorably degenerate toward a state of greater disorder, greater entropy. Yet all around us we see magnificent structures—galaxies, cells, ecosystems, human beings—that have all somehow managed to assemble themselves.”
The common argument used to explain this is that, locally, entropy can be lowered by external action, e.g. solar heating action, and that this applies to machines, such as a refrigerator, where the entropy in the cold chamber is being reduced, to growing crystals, and to living organisms. This local increase in order is, however, only possible at the expense of an entropy increase in the surroundings; here more disorder must be created. The conditioner of this statement suffices that living systems are open system
Open system
Open system may refer to:*Open system , one of a class of computers and associated software that provides some combination of interoperability, portability and open software standards, particularly Unix and Unix-like systems...
s in which both heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
, and or work
Mechanical work
In physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
may transfer into or out of the system. Unlike temperature, the putative entropy of a living system would drastically change if the organism were thermodynamically isolated. If an organism was in this type of “isolated” situation, its entropy would increase markedly as the once-living components of the organism decayed to an unrecognizable mass.
Phase change
Owing to these early developments, the typical example of entropy change ΔS is that associated with phase change. In solids, for example, which are typically ordered on the molecular scale, usually have smaller entropy than liquids, and liquids have smaller entropy than gases and colder gases have smaller entropy than hotter gases. Moreover, according to the third law of thermodynamicsThird law of thermodynamics
The third law of thermodynamics is a statistical law of nature regarding entropy:For other materials, the residual entropy is not necessarily zero, although it is always zero for a perfect crystal in which there is only one possible ground state.-History:...
, at absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
temperature, crystalline structures are approximated to have perfect "order" and zero entropy. This correlation occurs because the numbers of different microscopic quantum energy states available to an ordered system are usually much smaller than the number of states available to a system that appears to be disordered.
From his famous 1896 Lectures on Gas Theory, Boltzmann diagrams the structure of a solid body, as shown above, by postulating that each molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
in the body has a "rest position". According to Boltzmann, if it approaches a neighbor molecule it is repelled by it, but if it moves farther away there is an attraction. This, of course was a revolutionary perspective in its time; many, during these years, did not believe in the existence of either atoms or molecules (see: history of the molecule
History of the molecule
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms....
). According to these early views, and others such as those developed by William Thomson
William Thomson, 1st Baron Kelvin
William Thomson, 1st Baron Kelvin OM, GCVO, PC, PRS, PRSE, was a mathematical physicist and engineer. At the University of Glasgow he did important work in the mathematical analysis of electricity and formulation of the first and second laws of thermodynamics, and did much to unify the emerging...
, if energy in the form of heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
is added to a solid, so to make it into a liquid or a gas, a common depiction is that the ordering of the atoms and molecules becomes more random and chaotic with an increase in temperature:
Thus, according to Boltzmann, owing to increases in thermal motion, whenever heat is added to a working substance, the rest position of molecules will be pushed apart, the body will expand, and this will create more molar-disordered distributions and arrangements of molecules. These disordered arrangements, subsequently, correlate, via probability arguments, to an increase in the measure of entropy.
Adiabatic demagnetization
In the quest for ultra-cold temperatures, a temperature lowering technique called adiabatic demagnetization is used, where atomic entropy considerations are utilized which can be described in order-disorder terms. In this process, a sample of solid such as chrome-alum salt, whose molecules are equivalent to tiny magnets, is inside an insulated enclosure cooled to a low temperature, typically 2 or 4 kelvins, with a strong magnetic fieldMagnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
being applied to the container using a powerful external magnet, so that the tiny molecular magnets are aligned forming a well-ordered "initial" state at that low temperature. This magnetic alignment means that the magnetic energy of each molecule is minimal. The external magnetic field is then reduced, a removal that is considered to be closely reversible. Following this reduction, the atomic magnets then assume random less-ordered orientations, owing to thermal agitations, in the "final" state:
The "disorder" and hence the entropy associated with the change in the atomic alignments has clearly increased. In terms of energy flow, the movement from a magnetically aligned state requires energy from the thermal motion of the molecules, converting thermal energy into magnetic energy. Yet, according to the second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
, because no heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
can enter or leave the container, due to its adiabatic insulation, the system should exhibit no change in entropy, i.e. ΔS = 0. The increase in disorder, however, associated with the randomizing directions of the atomic magnets represents an entropy increase? To compensate for this, the disorder (entropy) associated with the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of the specimen must decrease by the same amount. The temperature thus falls as a result of this process of thermal energy being converted into magnetic energy. If the magnetic field is then increased, the temperature rises and the magnetic salt has to be cooled again using a cold material such as liquid helium.
Difficulties with the term "disorder"
In recent years the long-standing use of term "disorder" to discuss entropy has met with some criticism.Whilst when considered at a microscopic level the term disorder may quite correctly suggest an increased range of accessible possibilities, confusion may be caused because at the macroscopic level of everyday perception a higher entropy state may appear more homogenous, more even or more smoothly mixed—apparently in diametric opposition to its description as being "more disordered". Thus for example there may be dissonance at equilibrium
Thermal equilibrium
Thermal equilibrium is a theoretical physical concept, used especially in theoretical texts, that means that all temperatures of interest are unchanging in time and uniform in space...
being equated with "perfect internal disorder"; or the mixing of milk in coffee from apparent chaos to uniformity being described as a transition from an ordered state into a disordered state.
It has to be stressed, therefore, that "disorder", as used in a thermodynamic sense, relates to a full microscopic description of the system, rather than its apparent macroscopic properties. Many popular chemistry textbooks in recent editions increasingly have tended to instead present entropy through the idea of energy dispersal
Entropy (energy dispersal)
The description of entropy as energy dispersal provides an introductory method of teaching the thermodynamic concept of entropy. In physics and physical chemistry, entropy has commonly been defined as a scalar measure of the disorder of a thermodynamic system...
, which is a dominant contribution to entropy in most everyday situations.
See also
- EntropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
- History of entropyHistory of entropyThe concept of entropy developed in response to the observation that a certain amount of functional energy released from combustion reactions is always lost to dissipation or friction and is thus not transformed into useful work...
- Entropy (energy dispersal)Entropy (energy dispersal)The description of entropy as energy dispersal provides an introductory method of teaching the thermodynamic concept of entropy. In physics and physical chemistry, entropy has commonly been defined as a scalar measure of the disorder of a thermodynamic system...
- Entropy (statistical views)Entropy (statistical views)In classical statistical mechanics, the entropy function earlier introduced by Clausius is changed to statistical entropy using probability theory...
- Entropy (thermodynamic views)Entropy (thermodynamic views)In thermodynamics, entropy is a measure of how much of the energy of a system is potentially available to do work and how much of it is potentially manifest as heat. In classical thermodynamics, the entropy is defined only for a system in thermodynamic equilibrium...
External links
- Lambert, F.L. Entropy Sites — A Guide
- Lambert, F.L. Shuffled Cards, Messy Desks, and Disorderly Dorm Rooms - Examples of Entropy Increase? Nonsense! Journal of Chemical Education

