
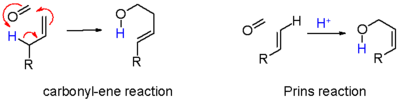
Prins reaction
Encyclopedia
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
consisting of an electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
to an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
or alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
followed by capture of a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
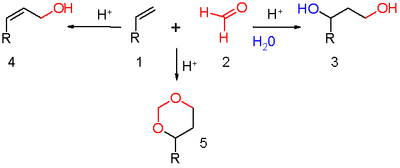
. The outcome of the reaction depends on reaction conditions (scheme 1). With water and a protic acid such as sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
as the reaction medium and formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
the reaction product is a 1,3-diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
. When water is absent dehydration
Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
takes place to an allyl alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
. With an excess of formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
and a low reaction temperature the reaction product is a dioxane. When water is replaced by acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
the corresponding esters are formed.
History
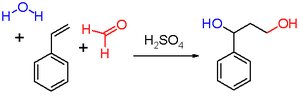
The original reactants employed by Dutch chemistChemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
Hendrik Jacobus Prins in his 1919 publication were styrene (scheme 2), pinene
Pinene
Pinene is a bicyclic monoterpene chemical compound. There are two structural isomers of pinene found in nature: α-pinene and β-pinene. As the name suggests, both forms are important constituents of pine resin; they are also found in the resins of many other conifers, as well as in non-coniferous...
, camphene
Camphene
Camphene is bicyclic monoterpene. It is nearly insoluble in water, but very soluble in common organic solvents. It volatilizes readily at room temperature and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil,...
, eugenol
Eugenol
Eugenol is a phenylpropene, an allyl chain-substituted guaiacol. Eugenol is a member of the phenylpropanoids class of chemical compounds. It is a clear to pale yellow oily liquid extracted from certain essential oils especially from clove oil, nutmeg, cinnamon, basil and bay leaf. It is slightly...
, isosafrole
Isosafrole
Isosafrole is a phenylpropene, a type of aromatic organic chemical with a smell similar to anise or licorice. It is found in small amounts in various essential oils, but is most commonly obtained by isomerizing the plant oil safrole....
and anethole
Anethole
Anethole is a phenylpropene, a type of aromatic compound that occurs widely in nature, in essential oils...
.
Synthetic rubber
Synthetic rubber is is any type of artificial elastomer, invariably a polymer. An elastomer is a material with the mechanical property that it can undergo much more elastic deformation under stress than most materials and still return to its previous size without permanent deformation...
.
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for this reaction is depicted in scheme 5. The carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
reactant (2) is protonated
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
by a protic acid and for the resulting oxonium ion
Oxonium ion
The oxonium ion in chemistry is any oxygen cation with three bonds. The simplest oxonium ion is the hydronium ion H3O+. Another oxonium ion frequently encountered in organic chemistry is obtained by protonation or alkylation of a carbonyl group e.g...
3 two resonance structures can be drawn. This electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
engages in an electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
with the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
to the carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
ic intermediate 4. Exactly how much positive charge is present on the secondary carbon atom in this intermediate should be determined for each reaction set. Evidence exists for neighbouring group participation
Neighbouring group participation
Neighbouring group participation or NGP in organic chemistry has been defined by IUPAC as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a sigma bond or pi bond . When NGP is in operation it is normal for the reaction rate to be increased...
of the hydroxyl oxygen or its neighboring carbon atom. When the overall reaction has a high degree of concertedness
Concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not to depend on solvent polarity ruling out large buildup...
, the charge built-up will be modest.
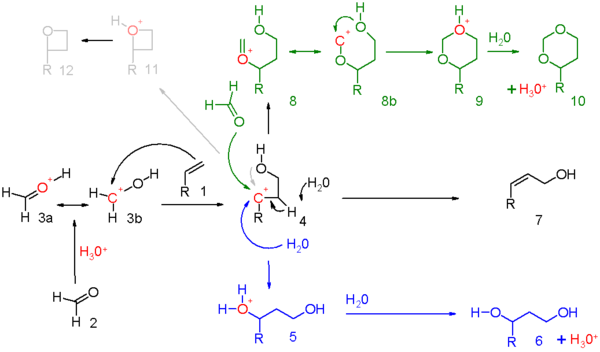
- in blue: capture of the carbocation by water or any suitable nucleophile through 5 to the 1,3-adduct 6.
- in black: proton abstraction in an elimination reactionElimination reactionAn elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
to unsaturated compound 7. When the alkene carries a methylene group, elimination and addition can be concerted with transfer of an allyl proton to the carbonyl group which in effect is an ene reactionEne reactionThe Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the...
in scheme 6.
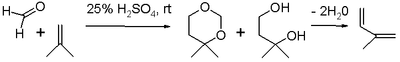
- in green: capture of the carbocation by additional carbonyl reactant. In this mode the positive charge is dispersed over oxygen and carbon in the resonance structures 8a and 8b. Ring closure leads through intermediate 9 to the dioxane 10. An example is the conversion of styrene to 4-phenyl-m-dioxane.
- in gray: only in specific reactions and when the carbocation is very stable the reaction takes a shortcut to the oxetaneOxetaneOxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
12. The photochemical Paternò–Büchi reaction between alkenes and aldehydes to oxetanes is more straightforward.
Variations
Many variations of the Prins reaction exist because it lends itself easily to cyclization reactions and because it is possible to capture the oxo-carbenium ion with a large array of nucleophiles.The halo-Prins reaction is one such modification with replacement of protic acids and water by lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s such as stannic chloride and boron tribromide
Boron tribromide
Boron tribromide, BBr3, is a colorless, fuming liquid compound containing boron and bromine. It is usually made by heating boron trioxide with carbon in the presence of bromine: this generates free boron which reacts vigorously with the bromine...
. The halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
is now the nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
recombining with the carbocation. The cyclization of certain allyl pulegones in scheme 7 with titanium tetrachloride
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
in dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
at -78°C gives access to the decalin skeleton with the hydroxyl group and chlorine group predominantly in cis configuration (91% cis). This observed cis diastereoselectivity is due to the intermediate formation of a trichlorotitanium alkoxide making possible an easy delivery of chlorine to the carbocation ion from the same face. The trans isomer is preferred (98% cis) when the switch is made to a tin tetrachloride reaction at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
.

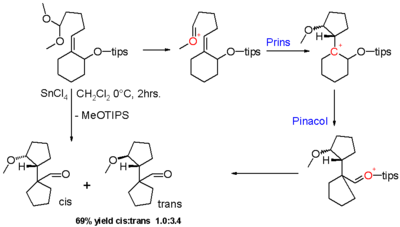
Cascade reaction
A cascade reaction or tandem reaction or domino reaction is a consecutive series of intramolecular organic reactions which often proceed via highly reactive intermediates. It allows the organic synthesis of complex multinuclear molecules from a single acyclic precursor. The substrate contains many...
of a Prins reaction and a pinacol rearrangement
Pinacol rearrangement
The pinacol rearrangement or pinacol-pinacolone rearrangement is a method for converting a 1,2-diol to a carbonyl compound in organic chemistry. This 1,2-rearrangement takes place under acidic conditions...
. The carbonyl group in the reactant in scheme 8 is masked as a dimethyl acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
and the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group is masked as a triisopropylsilyl ether
Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
(TIPS). With lewis acid stannic chloride the oxonium ion
Oxonium ion
The oxonium ion in chemistry is any oxygen cation with three bonds. The simplest oxonium ion is the hydronium ion H3O+. Another oxonium ion frequently encountered in organic chemistry is obtained by protonation or alkylation of a carbonyl group e.g...
is activated and the pinacol rearrangement of the resulting Prins intermediate results in ring contraction and referral of the positive charge to the TIPS ether which eventually forms an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
group in the final product as a mixture of cis and trans isomers with modest diastereoselectivity.
Uses
The Prins reaction is used in total synthesisTotal synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of complex natural products, for example, in a key step of that of the synthesis of exiguolide:

External links
- Prins reaction in Alkaloid total synthesis Link
- Prins reaction @ organic-chemistry.org

