Oxonium ion
Encyclopedia
The oxonium ion in chemistry
is any oxygen
cation with three bonds
. The simplest oxonium ion is the hydronium
ion H3O+. Another oxonium ion frequently encountered in organic chemistry
is obtained by protonation
or alkylation
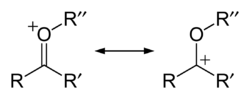
of a carbonyl
group e.g. R-C=O+-R' which forms a resonance structure with the fully fledged carbocation
R-C+-O-R' and is therefore especially stable:
Stable alkyloxonium salts exist; they are extensively used as alkylating agents. For example, triethyloxonium tetrafluoroborate is a white crystalline solid. It is a powerful ethylating agent. It can be used, for example, to produce ethyl esters when the conditions of traditional Fischer esterification are unsuitable.
Other hydrocarbon oxonium ions are formed by protonation or alkylation of alcohol
s or ethers . In acidic media, the oxonium functional group
produced by protonating an alcohol can be a leaving group
in the E2 elimination
reaction, because when it receives an electron, it becomes a water
molecule. The product is an alkene
. Extreme acidity, heat and dehydrating conditions are usually required.
Oxatriquinane
and oxatriquinacene are unusually stable oxonium ions, first described in 2008. Oxatriquinane does not react with boiling water or with alcohols, thiols, halide ions, or amines, although it does react with stronger nucleophile
s such as hydroxide
, cyanide
, and azide
.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
is any oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
cation with three bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
. The simplest oxonium ion is the hydronium
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
ion H3O+. Another oxonium ion frequently encountered in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is obtained by protonation
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
or alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group e.g. R-C=O+-R' which forms a resonance structure with the fully fledged carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
R-C+-O-R' and is therefore especially stable:
Stable alkyloxonium salts exist; they are extensively used as alkylating agents. For example, triethyloxonium tetrafluoroborate is a white crystalline solid. It is a powerful ethylating agent. It can be used, for example, to produce ethyl esters when the conditions of traditional Fischer esterification are unsuitable.
 |
 |
 |
 |
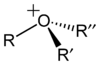
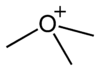
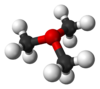
oxonium ion |
Skeletal formula The skeletal formula of an organic compound is a shorthand representation of its molecular structure, developed by the organic chemist, Friedrich August Kekulé von Stradonitz. Skeletal formulae are ubiquitous in organic chemistry, because they are relatively quick and simple to draw. Carbon and... of the trimethyloxonium cation |
Ball-and-stick model In chemistry, the ball-and-stick model is a molecular model of a chemical substance which is to display both the three-dimensional position of the atoms and the bonds between them... of trimethyloxonium |
Space-filling model In chemistry a space-filling model, also known as calotte model, is a type of three-dimensional molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic... of trimethyloxonium |
Other hydrocarbon oxonium ions are formed by protonation or alkylation of alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s or ethers . In acidic media, the oxonium functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
produced by protonating an alcohol can be a leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
in the E2 elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
reaction, because when it receives an electron, it becomes a water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
molecule. The product is an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. Extreme acidity, heat and dehydrating conditions are usually required.
Oxatriquinane
Oxatriquinane
Oxatriquinane is an alkyl oxonium ion with formula , remarkable for being stable in aqueous solution. It has a cyclononane backbone, with the trivalent oxygen connected to carbons 1,4, and 7, forming three fused pentagonal cycles....
and oxatriquinacene are unusually stable oxonium ions, first described in 2008. Oxatriquinane does not react with boiling water or with alcohols, thiols, halide ions, or amines, although it does react with stronger nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s such as hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
, cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
, and azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
.