Pinacol rearrangement
Encyclopedia
The pinacol rearrangement or pinacol-pinacolone rearrangement is a method for converting a 1,2-diol
to a carbonyl
compound in organic chemistry
. This 1,2-rearrangement
takes place under acidic conditions. The name of the reaction comes from the rearrangement of pinacol
to pinacolone
.
This reaction was first described by Wilhelm Rudolph Fittig
in 1860.
, protonation of one of the -OH groups occurs and a carbocation
is formed. If both the -OH groups are not alike, then the one which yields a more stable carbocation participates in the reaction. Subsequently, an alkyl group from the adjacent carbon migrates to the carbocation center. The driving force for this rearrangement step is believed to be the relative stability of the resultant oxonium ion, which has complete octet configuration at all centers (as opposed to the preceding carbocation). The migration of alkyl groups
in this reaction occurs in accordance with their usual migratory aptitude, i.e. hydride > Ph- > tertiary > secondary > methyl .
of the diol plays a crucial role in deciding the major product. An alkyl group which is situated trans- to the leaving -OH group alone may migrate. If otherwise, ring expansion occurs, i.e. the ring carbon itself migrates to the carbocation centre. This reveals another interesting feature of the reaction, viz. that it is largely concerted. There appears to be a connection between the migration origin and migration terminus throughout the reaction.
Moreover, if the migrating alkyl group has a chiral center as its key atom, the configuration at this center is retained even after migration takes place.
who correctly identified the reaction products involved.
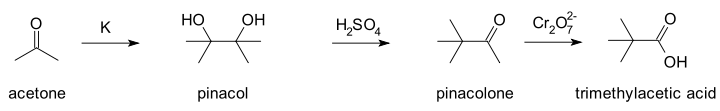
In a 1859 publication Wilhelm Rudolph Fittig
described the reaction of acetone
with potassium
metal... Fittig wrongly assumed a molecular formula of (C3H3O)n for acetone, the result of a long standing atomic weight debate finally settled at the Karlsruhe Congress
in 1860. He also wrongly believed acetone to be an alcohol which he hoped to prove by forming a metal alkoxide salt. The reaction product he obtained instead he called paraceton which he believed to be a acetone dimer. In his second publication in 1860 he reacted paraceton with sulfuric acid
(the actual pinacol rearrangement).
Again Fittig was unable to assign a molecular structure to the reaction product which he assumed to be another isomer or a polymer. Contemporary chemists who had already adapted to the new atomic weight reality did not fare better. One of them, Charles Friedel
, believed the reaction product to be the epoxide
tetramethylethylene oxide in analogy with reactions of ethylene glycol
. Finally Butlerov in 1873 came up with the correct structures after he independently synthesised the compound trimethylacetic acid which Friedel had obtained earlier by oxidizing with a dichromate.
Some of the problems during the determination of the structure are because carbon skeletal rearrangements were unknown at that time and therefore the new concept had to be found. Butlerov theory allowed the structure of carbon atoms in the molecule to rearrange and with this concept a structure for pinacolone could be found.
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
to a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compound in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. This 1,2-rearrangement
1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible...
takes place under acidic conditions. The name of the reaction comes from the rearrangement of pinacol
Pinacol
Pinacol is a white solid organic compound.-Preparation:It may be produced by the pinacol coupling reaction from acetone:-Reactions:As a vicinal-diol, it can rearrange to pinacolone by the pinacol rearrangement, e.g...
to pinacolone
Pinacolone
Pinacolone is an important ketone in organic chemistry. It has an odour reminiscent of peppermint and was discovered in 1866...
.
This reaction was first described by Wilhelm Rudolph Fittig
Wilhelm Rudolph Fittig
Wilhelm Rudolph Fittig was a German chemist. Fittig discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. He studied the action of sodium on ketones and hydrocarbons...
in 1860.
An overview of mechanism(discussion)
In the course of this organic reactionOrganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
, protonation of one of the -OH groups occurs and a carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
is formed. If both the -OH groups are not alike, then the one which yields a more stable carbocation participates in the reaction. Subsequently, an alkyl group from the adjacent carbon migrates to the carbocation center. The driving force for this rearrangement step is believed to be the relative stability of the resultant oxonium ion, which has complete octet configuration at all centers (as opposed to the preceding carbocation). The migration of alkyl groups
in this reaction occurs in accordance with their usual migratory aptitude, i.e. hydride > Ph- > tertiary > secondary > methyl .
Stereochemistry of the rearrangement
In cyclic systems, the reaction presents more features of interest. In these reactions, the stereochemistryStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of the diol plays a crucial role in deciding the major product. An alkyl group which is situated trans- to the leaving -OH group alone may migrate. If otherwise, ring expansion occurs, i.e. the ring carbon itself migrates to the carbocation centre. This reveals another interesting feature of the reaction, viz. that it is largely concerted. There appears to be a connection between the migration origin and migration terminus throughout the reaction.
Moreover, if the migrating alkyl group has a chiral center as its key atom, the configuration at this center is retained even after migration takes place.
History
Although Fittig first published about the pinacol rearrangement,it was not Fittig but Aleksandr ButlerovAleksandr Butlerov
Aleksandr Mikhailovich Butlerov was a Russian chemist, one of the principal creators of the theory of chemical structure , the first to incorporate double bonds into structural formulas, the discoverer of hexamine , and the discoverer of the formose reaction.The...
who correctly identified the reaction products involved.
In a 1859 publication Wilhelm Rudolph Fittig
Wilhelm Rudolph Fittig
Wilhelm Rudolph Fittig was a German chemist. Fittig discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. He studied the action of sodium on ketones and hydrocarbons...
described the reaction of acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
with potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
metal... Fittig wrongly assumed a molecular formula of (C3H3O)n for acetone, the result of a long standing atomic weight debate finally settled at the Karlsruhe Congress
Karlsruhe Congress
The Karlsruhe Congress was an international meeting of chemists held in Karlsruhe, Germany from 3 to 5 September, 1860. It was the first international conference of chemistry worldwide.- The meeting :...
in 1860. He also wrongly believed acetone to be an alcohol which he hoped to prove by forming a metal alkoxide salt. The reaction product he obtained instead he called paraceton which he believed to be a acetone dimer. In his second publication in 1860 he reacted paraceton with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
(the actual pinacol rearrangement).
Again Fittig was unable to assign a molecular structure to the reaction product which he assumed to be another isomer or a polymer. Contemporary chemists who had already adapted to the new atomic weight reality did not fare better. One of them, Charles Friedel
Charles Friedel
Charles Friedel was a French chemist and mineralogist. A native of Strasbourg, France, he was a student of Louis Pasteur at the Sorbonne...
, believed the reaction product to be the epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
tetramethylethylene oxide in analogy with reactions of ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
. Finally Butlerov in 1873 came up with the correct structures after he independently synthesised the compound trimethylacetic acid which Friedel had obtained earlier by oxidizing with a dichromate.
Some of the problems during the determination of the structure are because carbon skeletal rearrangements were unknown at that time and therefore the new concept had to be found. Butlerov theory allowed the structure of carbon atoms in the molecule to rearrange and with this concept a structure for pinacolone could be found.
See also
- semipinacol rearrangementSemipinacol rearrangementThe semipinacol rearrangement is a rearrangement reaction in organic chemistry involving a heterosubstituted alcohol of the type R1R2C-CR3R4. The hetero substituent can be a halogen , a tosylate, a mesylate or a thioxy group. This reaction proceeds by removal of the leaving group X forming a...
- Tiffeneau-Demjanov rearrangementTiffeneau-Demjanov rearrangementThe Tiffeneau-Demjanov rearrangement is the chemical reaction of a 1-aminomethyl-cycloalkanol with nitrous acid to form an enlarged cycloketone....
, in which the leaving group is a diazo (from amine) rather than oxonium (from hydroxyl)