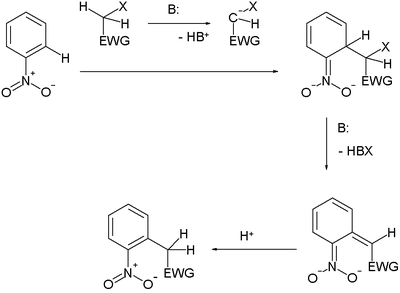
Vicarious nucleophilic substitution
Encyclopedia
Vicarious nucleophilic substitution in organic chemistry
is a special type of nucleophilic aromatic substitution
in which a nucleophile
replaces hydrogen and not an aromatic substituent like a halogen that is ordinarily encountered in this reaction type. This reaction type was reviewed in 1987 by Polish chemists Mieczysław Mąkosza
and Jerzy Winiarski.
It is typically encountered with nitroarenes
and especially with carbon nucleophiles, resulting in new alkylated arenes. Carbon nucleophiles carry a electron-withdrawing group and a leaving group
:
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a special type of nucleophilic aromatic substitution
Nucleophilic aromatic substitution
right|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
in which a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
replaces hydrogen and not an aromatic substituent like a halogen that is ordinarily encountered in this reaction type. This reaction type was reviewed in 1987 by Polish chemists Mieczysław Mąkosza
Mieczysław Mąkosza
Mieczysław Józef Mąkosza is a Polish chemist specializing in organic synthesis and investigation of organic mechanisms. Along with Jerzy Winiarski he is credited for the discovery of the aromatic vicarious nucleophilic substitution, VNS. He also contributed to the discovery of phase transfer...
and Jerzy Winiarski.
It is typically encountered with nitroarenes
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
and especially with carbon nucleophiles, resulting in new alkylated arenes. Carbon nucleophiles carry a electron-withdrawing group and a leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
: