ANRORC mechanism
Encyclopedia
The ANRORC mechanism in organic chemistry
describes a special type of substitution reaction
. ANRORC stands for Addition of the Nucleophile, Ring Opening, and Ring Closure in nucleophilic attack on ring systems and it helps to explain product formation and distribution in some nucleophilic substitution
s especially in heterocyclic compound
s.
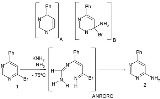
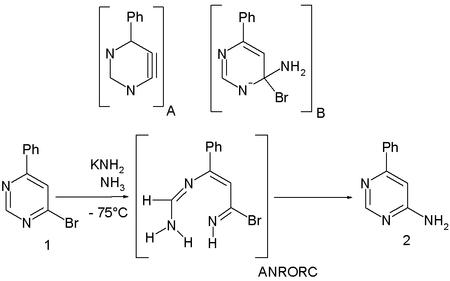
This reaction mechanism
has been extensively studied in reactions of metal amide nucleophiles (such as sodium amide
) and substituted pyrimidine
s (for instance 4-phenyl-6-bromopyrimidine 1) in ammonia
at low temperatures. The main reaction product is 4-phenyl-6-aminopyrimidine 2 with the bromine
substituent
replaced by an amine
. This rules out the formation of an aryne
intermediate A which would also give the 5-substituted isomer.
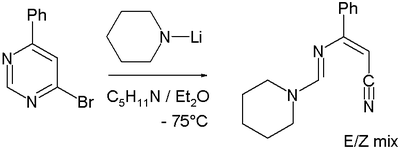
 The exclusion of a second intermediate in this reaction, the Meisenheimer complex
The exclusion of a second intermediate in this reaction, the Meisenheimer complex
B in favor of the ring-opened ANRORC intermediate is based on several pieces of evidence. With other amines such as piperidine
the ring-opened compound after loss of hydrogen bromide
to the nitrile
is also the isolated reaction product:
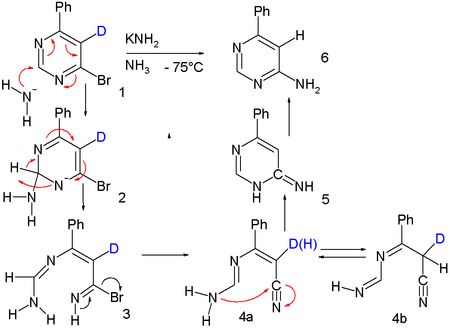
 More evidence is gained by isotope labeling with deuterium
More evidence is gained by isotope labeling with deuterium
at C5:
The deuterium atom is no longer present in the reaction product and this is again explained by the ANRORC mechanism where the ring-opened intermediate 4 is a tautomer
ic pair enabling fast H-D exchange:
 The final piece of evidence is provided by an isotope scrambling experiment with both nitrogen atoms in the pyrimidine core replaced by the 14N isotope
The final piece of evidence is provided by an isotope scrambling experiment with both nitrogen atoms in the pyrimidine core replaced by the 14N isotope
to a degree of 3% each:
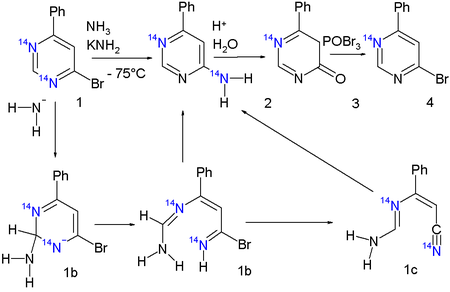 In the final product 4 (reversed to the reactant, after acid hydrolysis and bromination of 2) roughly half the isotope content is lost, clearly demonstrating that one internal nitrogen atom has been displaced to an external nitrogen atom.
In the final product 4 (reversed to the reactant, after acid hydrolysis and bromination of 2) roughly half the isotope content is lost, clearly demonstrating that one internal nitrogen atom has been displaced to an external nitrogen atom.
The Zincke reaction
is a named reaction involving an ANRORC mechanism.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
describes a special type of substitution reaction
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
. ANRORC stands for Addition of the Nucleophile, Ring Opening, and Ring Closure in nucleophilic attack on ring systems and it helps to explain product formation and distribution in some nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
s especially in heterocyclic compound
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
s.
This reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
has been extensively studied in reactions of metal amide nucleophiles (such as sodium amide
Sodium amide
Sodium amide, commonly called sodamide, is the chemical compound with the formula NaNH2. This solid, which is dangerously reactive toward water, is white when pure, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process...
) and substituted pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
s (for instance 4-phenyl-6-bromopyrimidine 1) in ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
at low temperatures. The main reaction product is 4-phenyl-6-aminopyrimidine 2 with the bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
replaced by an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
. This rules out the formation of an aryne
Aryne
In chemistry, an aryne is an uncharged reactive intermediate derived from an aromatic system by removal of two ortho substituents, leaving two orbitals with two electrons distributed between them....
intermediate A which would also give the 5-substituted isomer.

Meisenheimer complex
A Meisenheimer complex or Jackson-Meisenheimer complex in organic chemistry is a 1:1 reaction adduct between an arene carrying electron withdrawing groups and nucleophile...
B in favor of the ring-opened ANRORC intermediate is based on several pieces of evidence. With other amines such as piperidine
Piperidine
Piperidine is an organic compound with the molecular formula 5NH. This heterocyclic amine consists of a six-membered ring containing five methylene units and one nitrogen atom...
the ring-opened compound after loss of hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
to the nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
is also the isolated reaction product:

Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
at C5:
The deuterium atom is no longer present in the reaction product and this is again explained by the ANRORC mechanism where the ring-opened intermediate 4 is a tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
ic pair enabling fast H-D exchange:

Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
to a degree of 3% each:

The Zincke reaction
Zincke reaction
The Zincke reaction is an organic reaction in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine, named after Theodor Zincke....
is a named reaction involving an ANRORC mechanism.

