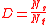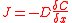Metal oxide adhesion
Encyclopedia
The strength of metal oxide adhesion effectively determines the wetting
of the metal-oxide interface. The strength of this adhesion is important, for instance, in production of light bulbs and fiber-matrix composites
that depend on the optimization of wetting to create metal-ceramic interfaces The strength of adhesion also determines the extent of dispersion on catalytically
active metal.
Metal oxide adhesion is important for applications like complementary metal oxide semiconductor
devices. These devices make possible the high packing densities of modern integrated circuit
s.
of metal oxidation reactions is in the O2(g) because the gas
eous oxygen molecules have translation entropy which is not present in the solid
phase. For this reason, the change in entropy (ΔS) for oxidation is most always negative because this reaction generates less disorder by creating a solid oxide layer from the solid metal and gaseous oxygen. The standard state change of enthalpy is relatively independent and thus the gradient of the change in Gibbs free energy
as a function of temperature is linear. This dictates that an oxide becomes less thermodynamically
stable with increasing temperature.
An important distinction between equilibrium
wetting and non-equilibrium wetting is that the non-equilibrium condition occurs when a chemical reaction is taking place. This non-equilibrium wetting is an irreversible thermodynamic process that accounts for the changes of the chemical potential
when forming a new boundary phase, such as an oxide.
work needed to separate the interface into two free surfaces. Important as a state function depending on the mechanical properties. It is referred to as ideal because when the two free surfaces create an interface
, the concentration of the interface will only be identical to the bulk at the instant the surface is created. In order to reach chemical equilibrium, the process of diffusion
will take place which will increase any measurement of the work of separation. The work of adhesion is the reversible free energy
change for making free surfaces from interfaces. It is represented by the equation:
where:
Wad is the work of adhesion
γm and γo are the respective surface energies of the metal and oxide
γmo is the surface energy between the two materials in contact
The following table gives some common metals and their corresponding surface energies. All the metals are face-centered cubic
crystal structure and these surface energies correspond to the (100) surface plane.
s are generated according to the second law of thermodynamics
and are a graphical representation of the change in the Gibbs free energy with respect to changing temperature for the formation of oxides.
, but their microscopic heterogeneity
plays a crucial role in the relationship between the metal and its oxide.
allow for multiple oxides to be formed from the same two elements
. As the composition of the materials changes through diffusion, different oxides are able to stack up on one another. The adhesion in this situation involves the metal-oxide and oxide-oxide interfaces which add increasing complexity to the mechanics.
where:
E is the binding energy of the material
T is the temperature of the system
S is the surface entropy of the material
The binding energy favors a smoother surface that minimizes the number of danging bonds, while the surface entropy term favors a rougher surface with increasing dangling bonds as the temperature is increased.
, but individual Bravais lattice planes, defined by their Miller indices, may be non-polar or polar based on their symmetry. A dipole moment increases the surface gibbs free energy, but the greater polarizability
of oxygen ions as compared to metals allows polarization to decrease the surface energy and thus increase the ability of metals to form oxides. Consequently, different exposed metal faces may adhere weakly to non-polar oxide faces, but be able to perfectly wet a polar face.
are the localized fluctuations of surface electronic states and binding energies. Surface reactions, adsorption, and nucleation can be drastically affected by the presence of these defects.
Oxide growth is dependent upon the flux (diffusion
) of either coupled or independent anions and cations through the oxide layer. Stoichiometric oxides have an integer ratio of atoms can only support coupled diffusion of anions and cations through the lattice migration of Schottky defect
s (paired anion/cation vacancies) or Frenkel defect
s (complete anion lattice with cation vacancies and intersticials
). Non-stoichiometric oxide films support independent ion diffusion and are either n-type
(extra electrons) or p-type
(extra electron holes). Although there are only two valence states, there are three types:
Non-stoichiometric oxides most commonly have excess metal cations as a result of insufficient oxygen during the creation of the oxide layer. Excess metal atoms with a smaller radius than O2- anions are ionized within the crystal lattice as interstitial defects and their lost electrons remain free within the crystal, not taken by the oxygen atoms. The presence of mobile electrons within the crystal lattice significantly contributes to the conduction of electricity and the mobility of ions.
Impurity
elements in the material can have a large effect on the adhesion of oxide films. When the impurity element increases the adherence of the oxide to the metal it is known as the reactive element effect or RE effect. Many mechanics theories exist on this topic. The majority of them attribute the increase in adhesion strength to the greater thermodynamic stability of the impurity element bonded with oxygen than the metal bonded to the oxygen. Inserting yttrium into nickel alloys to strengthen the oxide adhesion is an example of the reactive element effect.
Dislocation
s are thermodynamically unstable, kinetically trapped defects. Surface dislocations often create a screw dislocation when stress is applied. In certain cases, screw dislocations can negate the nucleation
energy barrier for crystal growth.
of gas atoms is either commensurate or incommensurate. Commensurate adsorption is defined by having a crystal structure relationship between substrate-adsorbate layer that produces a coherent interface. Wood's notation is a description of the relationship between the simplest repeating unit area of the solid and adsorbate. The difference between the resulting commensurate interfaces can be described as an effect of misfit. The interfacial interaction can be modeled as the sg plus the stored elastic displacement energy due to lattice misfit. A large misfit corresponds to an incoherent interface where there is no coherency strain and the interface energy can be taken as simply the
sg plus the stored elastic displacement energy due to lattice misfit. A large misfit corresponds to an incoherent interface where there is no coherency strain and the interface energy can be taken as simply the  sg. In contrast, a small misfit corresponds with a coherent interface and coherency strain that results in the interfacial energy equivalent to the minimum
sg. In contrast, a small misfit corresponds with a coherent interface and coherency strain that results in the interfacial energy equivalent to the minimum 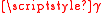 sg.
sg.
to GPa stresses. The cause of this huge range stems from multiple phenomena dealing with at least four different types of adhesion
. The main types of bonding that form adhesion are electrostatic, dispersive (van der Waals
or London forces), chemical and diffusive bonding. As the adhesive forces increase, separation in crystalline materials can go from elastic debonding to elasic-plastic debonding. This is due to a larger number of bonds being formed or an increase in strength of the bonds between the two materials. Elastic-plastic debonding is when local stresses are high enough to move dislocations or make new ones.
of oxide growth. If there molecule is absorbed there are three potential outcomes. The surface interaction can be strong enough to dissociate the gas molecule into separate atoms or constituents. The molecule may also react with surface atoms to change its chemical properties. The third possibility is solid surface catalysis, a binary chemical reaction with a previously adsorbed molecule on the surface.
in general can be modeled by:
where:
Ns is the number of atoms on the surface
Nt is the total number of atoms in the material
Dispersion is crucial to the growth of oxides because only atoms that are exposed to the interface can react to form oxides.
where:
J is the flux
and has units of mol·m−2·s−1
D is the diffusivity of the ions in the material
δC is the change in concentration of the material
δx is the thickness of the oxide layer
in chemistry was awarded to Gerhard Ertl
for the study of solid-gas interface molecular processes. One such process is the oscillatory kinetic catalysis. Oscillatory kinetic catalysis can be explained by different crystal surfaces favoring unmodified faces and reconstruction to reduce surface strain. The presence of CO can cause the reversal of surface reconstruction past a certain percent coverage. Once the reversal occurs, oxygen can be chemisorbed on the reverted surfaces. This produces a adsorption pattern with areas of surface coverage rich in CO and others O2.
is determined by the difference between the unprimed equilibrium and the instantaneous interfacial free energies.
Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
of the metal-oxide interface. The strength of this adhesion is important, for instance, in production of light bulbs and fiber-matrix composites
Composite material
Composite materials, often shortened to composites or called composition materials, are engineered or naturally occurring materials made from two or more constituent materials with significantly different physical or chemical properties which remain separate and distinct at the macroscopic or...
that depend on the optimization of wetting to create metal-ceramic interfaces The strength of adhesion also determines the extent of dispersion on catalytically
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
active metal.
Metal oxide adhesion is important for applications like complementary metal oxide semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
devices. These devices make possible the high packing densities of modern integrated circuit
Integrated circuit
An integrated circuit or monolithic integrated circuit is an electronic circuit manufactured by the patterned diffusion of trace elements into the surface of a thin substrate of semiconductor material...
s.
Oxide Thermodynamics
The majority of the entropyEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
of metal oxidation reactions is in the O2(g) because the gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous oxygen molecules have translation entropy which is not present in the solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
phase. For this reason, the change in entropy (ΔS) for oxidation is most always negative because this reaction generates less disorder by creating a solid oxide layer from the solid metal and gaseous oxygen. The standard state change of enthalpy is relatively independent and thus the gradient of the change in Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
as a function of temperature is linear. This dictates that an oxide becomes less thermodynamically
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
stable with increasing temperature.
An important distinction between equilibrium
Equilibrium
Equilibrium is the condition of a system in which competing influences are balanced. The word may refer to:-Biology:* Equilibrioception, the sense of a balance present in human beings and other animals...
wetting and non-equilibrium wetting is that the non-equilibrium condition occurs when a chemical reaction is taking place. This non-equilibrium wetting is an irreversible thermodynamic process that accounts for the changes of the chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
when forming a new boundary phase, such as an oxide.
Work of Adhesion
The ideal work of separation Wsep is the reversibleReversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
work needed to separate the interface into two free surfaces. Important as a state function depending on the mechanical properties. It is referred to as ideal because when the two free surfaces create an interface
Interface (chemistry)
An interface is a surface forming a common boundary among two different phases, such as an insoluble solid and a liquid, two immiscible liquids or a liquid and an insoluble gas. The importance of the interface depends on which type of system is being treated: the bigger the quotient area/volume,...
, the concentration of the interface will only be identical to the bulk at the instant the surface is created. In order to reach chemical equilibrium, the process of diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
will take place which will increase any measurement of the work of separation. The work of adhesion is the reversible free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
change for making free surfaces from interfaces. It is represented by the equation:
where:
Wad is the work of adhesion
γm and γo are the respective surface energies of the metal and oxide
γmo is the surface energy between the two materials in contact
The following table gives some common metals and their corresponding surface energies. All the metals are face-centered cubic
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
crystal structure and these surface energies correspond to the (100) surface plane.
| Material | Surface Energy  |
|---|---|
| Al | 1.347 |
| Pb | 0.377 |
| Yb | 0.478 |
| Cu | 2.166 |
| Pd | 2.326 |
| Ag | 1.200 |
| Pt | 2.734 |
| Au | 1.627 |
Oxide Stability
Ellingham diagramEllingham diagram
An Ellingham diagram is a graph showing the temperature dependence of the stability for compounds. This analysis is usually used to evaluate the ease of reduction of metal oxides and sulphides. These diagrams were first constructed by Harold Ellingham in 1944...
s are generated according to the second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
and are a graphical representation of the change in the Gibbs free energy with respect to changing temperature for the formation of oxides.
Structure
Real surfaces may be macroscopically homogeneousHomogeneity and heterogeneity
Homogeneity and heterogeneity are concepts relating to the uniformity or lack thereof in a substance. A material that is homogeneous is uniform in composition or character; one that is heterogeneous lacks uniformity in one of these qualities....
, but their microscopic heterogeneity
Homogeneity and heterogeneity
Homogeneity and heterogeneity are concepts relating to the uniformity or lack thereof in a substance. A material that is homogeneous is uniform in composition or character; one that is heterogeneous lacks uniformity in one of these qualities....
plays a crucial role in the relationship between the metal and its oxide.
Transition Metal Oxides
Certain transition metals form multiple oxide layers that have different stoichiometric compositions. This is due to the metal having different valency states that have fewer or greater electrons in the valence shell. These different valency statesElectron shell
An electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" , followed by the "2 shell" , then the "3 shell" , and so on further and further from the nucleus. The shell letters K,L,M,.....
allow for multiple oxides to be formed from the same two elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
. As the composition of the materials changes through diffusion, different oxides are able to stack up on one another. The adhesion in this situation involves the metal-oxide and oxide-oxide interfaces which add increasing complexity to the mechanics.
Roughness
Increasing surface roughness increases the number of dangling bonds at the metal-oxide interface. The surface free energy of a crystal face is:where:
E is the binding energy of the material
T is the temperature of the system
S is the surface entropy of the material
The binding energy favors a smoother surface that minimizes the number of danging bonds, while the surface entropy term favors a rougher surface with increasing dangling bonds as the temperature is increased.
Heterogeneity
Solid adsorption of an oxygen molecule depends on the heterogeneity of the substrate. Crystalline solid adsorption is dependent on the exposed crystal faces, grain orientations, and inherent defects because these factors provide adsorption sites with different steric configurations. Adsorption is largely determined by the reduction of gibbs free energy associated with the exposed substrate.Crystallographic Orientation
A material's charge remains neutral when a surface is created by the law of charge conservationCharge conservation
In physics, charge conservation is the principle that electric charge can neither be created nor destroyed. The net quantity of electric charge, the amount of positive charge minus the amount of negative charge in the universe, is always conserved...
, but individual Bravais lattice planes, defined by their Miller indices, may be non-polar or polar based on their symmetry. A dipole moment increases the surface gibbs free energy, but the greater polarizability
Polarizability
Polarizability is the measure of the change in a molecule's electron distribution in response to an applied electric field, which can also be induced by electric interactions with solvents or ionic reagents. It is a property of matter...
of oxygen ions as compared to metals allows polarization to decrease the surface energy and thus increase the ability of metals to form oxides. Consequently, different exposed metal faces may adhere weakly to non-polar oxide faces, but be able to perfectly wet a polar face.
Defects
Surface defectsCrystallographic defect
Crystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
are the localized fluctuations of surface electronic states and binding energies. Surface reactions, adsorption, and nucleation can be drastically affected by the presence of these defects.
Vacancies
Oxide growth is dependent upon the flux (diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
) of either coupled or independent anions and cations through the oxide layer. Stoichiometric oxides have an integer ratio of atoms can only support coupled diffusion of anions and cations through the lattice migration of Schottky defect
Schottky defect
A Schottky defect is a type of point defect in a crystal lattice named after Walter H. Schottky.The defect forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid....
s (paired anion/cation vacancies) or Frenkel defect
Frenkel defect
The Frenkel Defect is shown by ionic solids. The smaller ion is displaced from its lattice position to an interstitial site. It creates a vacancy defect at its original site and an interstitial defect at its new location.-Definition:...
s (complete anion lattice with cation vacancies and intersticials
Interstitial defect
Interstitials are a variety of crystallographic defects, i.e. atoms which occupy a site in the crystal structure at which there is usually not an atom, or two or more atoms sharing one or more lattice sites such that the number of atoms is larger than the number of lattice sites.They are generally...
). Non-stoichiometric oxide films support independent ion diffusion and are either n-type
N-type semiconductor
N-type semiconductors are a type of extrinsic semiconductor where the dopant atoms are capable of providing extra conduction electrons to the host material . This creates an excess of negative electron charge carriers....
(extra electrons) or p-type
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
(extra electron holes). Although there are only two valence states, there are three types:
- cation excess (n-type)
- anion deficit (n-type)
- cation deficit (p-type)
Non-stoichiometric oxides most commonly have excess metal cations as a result of insufficient oxygen during the creation of the oxide layer. Excess metal atoms with a smaller radius than O2- anions are ionized within the crystal lattice as interstitial defects and their lost electrons remain free within the crystal, not taken by the oxygen atoms. The presence of mobile electrons within the crystal lattice significantly contributes to the conduction of electricity and the mobility of ions.
Impurities
Impurity
Impurity
Impurities are substances inside a confined amount of liquid, gas, or solid, which differ from the chemical composition of the material or compound.Impurities are either naturally occurring or added during synthesis of a chemical or commercial product...
elements in the material can have a large effect on the adhesion of oxide films. When the impurity element increases the adherence of the oxide to the metal it is known as the reactive element effect or RE effect. Many mechanics theories exist on this topic. The majority of them attribute the increase in adhesion strength to the greater thermodynamic stability of the impurity element bonded with oxygen than the metal bonded to the oxygen. Inserting yttrium into nickel alloys to strengthen the oxide adhesion is an example of the reactive element effect.
Dislocations
Dislocation
Dislocation
In materials science, a dislocation is a crystallographic defect, or irregularity, within a crystal structure. The presence of dislocations strongly influences many of the properties of materials...
s are thermodynamically unstable, kinetically trapped defects. Surface dislocations often create a screw dislocation when stress is applied. In certain cases, screw dislocations can negate the nucleation
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
energy barrier for crystal growth.
Oxide-Support Relationship
The adsorption of a monolayerMonolayer
- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail...
of gas atoms is either commensurate or incommensurate. Commensurate adsorption is defined by having a crystal structure relationship between substrate-adsorbate layer that produces a coherent interface. Wood's notation is a description of the relationship between the simplest repeating unit area of the solid and adsorbate. The difference between the resulting commensurate interfaces can be described as an effect of misfit. The interfacial interaction can be modeled as the
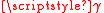 sg plus the stored elastic displacement energy due to lattice misfit. A large misfit corresponds to an incoherent interface where there is no coherency strain and the interface energy can be taken as simply the
sg plus the stored elastic displacement energy due to lattice misfit. A large misfit corresponds to an incoherent interface where there is no coherency strain and the interface energy can be taken as simply the 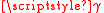 sg. In contrast, a small misfit corresponds with a coherent interface and coherency strain that results in the interfacial energy equivalent to the minimum
sg. In contrast, a small misfit corresponds with a coherent interface and coherency strain that results in the interfacial energy equivalent to the minimum 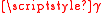 sg.
sg.Strength of Bond
The strength of the bond between the oxide and metal for the same nominal contact area can range from PaPascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
to GPa stresses. The cause of this huge range stems from multiple phenomena dealing with at least four different types of adhesion
Adhesion
Adhesion is any attraction process between dissimilar molecular species that can potentially bring them in close contact. By contrast, cohesion takes place between similar molecules....
. The main types of bonding that form adhesion are electrostatic, dispersive (van der Waals
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
or London forces), chemical and diffusive bonding. As the adhesive forces increase, separation in crystalline materials can go from elastic debonding to elasic-plastic debonding. This is due to a larger number of bonds being formed or an increase in strength of the bonds between the two materials. Elastic-plastic debonding is when local stresses are high enough to move dislocations or make new ones.
Solid-Gas Kinetics
When a gas molecule strikes a solid surface the molecule may either rebound or be adsorbed. The rate at which gas molecules strike the surface is a large factor in the overall kineticsChemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
of oxide growth. If there molecule is absorbed there are three potential outcomes. The surface interaction can be strong enough to dissociate the gas molecule into separate atoms or constituents. The molecule may also react with surface atoms to change its chemical properties. The third possibility is solid surface catalysis, a binary chemical reaction with a previously adsorbed molecule on the surface.
Dispersion
Most often it is kinetically favorable for the growth of a single oxide monolayer to be completed before the growth of subsequent layers. DispersionDispersion
Dispersion may refer to:In physics:*The dependence of wave velocity on frequency or wavelength:**Dispersion , for light waves**Dispersion **Acoustic dispersion, for sound waves...
in general can be modeled by:
where:
Ns is the number of atoms on the surface
Nt is the total number of atoms in the material
Dispersion is crucial to the growth of oxides because only atoms that are exposed to the interface can react to form oxides.
Diffusion
After the initial oxide monolayer is formed, new layers begin to build and the ions must be able to diffuse through the oxide in order to increase thickness of the oxide. The rate of oxidation is controlled by how fast these ions are able to diffuse through the material. As the thickness of the oxide increases, the rate of oxidation decreases because it requires the atoms to travel a further distance. This rate can quantified by calculating the rate of diffusion of vacancies or ions using Fick’s first law of diffusion.where:
J is the flux
Flux
In the various subfields of physics, there exist two common usages of the term flux, both with rigorous mathematical frameworks.* In the study of transport phenomena , flux is defined as flow per unit area, where flow is the movement of some quantity per time...
and has units of mol·m−2·s−1
D is the diffusivity of the ions in the material
δC is the change in concentration of the material
δx is the thickness of the oxide layer
Solid Surface Catalysis
In 2007 the Nobel PrizeNobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
in chemistry was awarded to Gerhard Ertl
Gerhard Ertl
Gerhard Ertl is a German physicist and a Professor emeritus at the Department of Physical Chemistry, Fritz-Haber-Institut der Max-Planck-Gesellschaft in Berlin, Germany...
for the study of solid-gas interface molecular processes. One such process is the oscillatory kinetic catalysis. Oscillatory kinetic catalysis can be explained by different crystal surfaces favoring unmodified faces and reconstruction to reduce surface strain. The presence of CO can cause the reversal of surface reconstruction past a certain percent coverage. Once the reversal occurs, oxygen can be chemisorbed on the reverted surfaces. This produces a adsorption pattern with areas of surface coverage rich in CO and others O2.
Driving Force
The driving force of catalysisCatalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
is determined by the difference between the unprimed equilibrium and the instantaneous interfacial free energies.
See also
- OxideOxideAn oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
- Crystallographic defectCrystallographic defectCrystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
- CorrosionCorrosionCorrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
- Oxidation potential
- Reduction potentialReduction potentialReduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts , or millivolts...
- Pourbaix diagramPourbaix diagramIn chemistry, a Pourbaix diagram, also known as a potential/pH diagram, maps out possible stable phases of an aqueous electrochemical system. Predominant ion boundaries are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes...
- Ellingham diagramEllingham diagramAn Ellingham diagram is a graph showing the temperature dependence of the stability for compounds. This analysis is usually used to evaluate the ease of reduction of metal oxides and sulphides. These diagrams were first constructed by Harold Ellingham in 1944...
- MOSFETMOSFETThe metal–oxide–semiconductor field-effect transistor is a transistor used for amplifying or switching electronic signals. The basic principle of this kind of transistor was first patented by Julius Edgar Lilienfeld in 1925...
- Metal oxide varistor
- Surface Properties of Transition Metal OxidesSurface properties of transition metal oxidesTransition metal oxides are compounds composed of oxygen atoms bound to transition metals. They are commonly utilized for their catalytic activity and semiconductive properties. Transition metal oxides are also frequently used as pigments in paints and plastics, most notably titanium dioxide...