Schottky defect
Encyclopedia
A Schottky defect is a type of point defect in a crystal lattice named after Walter H. Schottky
.
The defect forms when oppositely charged ion
s leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric
units, to maintain an overall neutral charge in the ionic solid. The vacancies are then free to move about as their own entities. Normally these defects will lead to a decrease in the density
of the crystal. The following are the chemical equations in Kröger-Vink Notation
for the formation of Schottky defects in TiO2 and BaTiO3.
Ø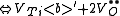
Ø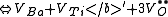
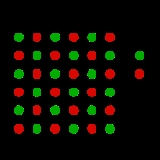
This can be illustrated schematically with a two-dimensional diagram of a sodium chloride
crystal lattice:
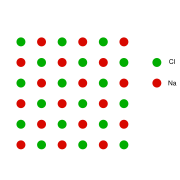
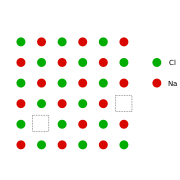
It is a vacancy defect (due to missing ions) and also a stoichiometric defect, as the ratio of the number of cations and anions remains the same.
Examples : NaCl, KCl, CsCl, KBr, AgCl. etc.
Experimental observations show that at room temperature in an NaCl crystal there is one Schottky defect per 1016 ions.
Walter H. Schottky
Walter Hermann Schottky was a German physicist who played a major early role in developing the theory of electron and ion emission phenomena, invented the screen-grid vacuum tube in 1915 and the pentode in 1919 while working at Siemens, and later made many significant contributions in the areas of...
.
The defect forms when oppositely charged ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
units, to maintain an overall neutral charge in the ionic solid. The vacancies are then free to move about as their own entities. Normally these defects will lead to a decrease in the density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
of the crystal. The following are the chemical equations in Kröger-Vink Notation
Kröger-Vink Notation
Kröger–Vink notation is set of conventions used to describe electric charge and lattice position for point defect species in crystals. It is primarily used for ionic crystals and is particularly useful for describing various defect reactions. It was proposed by F. A. Kröger and H. J. Vink.-General...
for the formation of Schottky defects in TiO2 and BaTiO3.
Ø
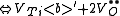
Ø
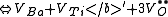
This can be illustrated schematically with a two-dimensional diagram of a sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
crystal lattice:


Definition
If in an ionic crystal of type A+B- an equal number of cations and anions are missing from their lattice sites so that electrical neutrality as well as stoichiometry is maintained this is called a Schottky Defect.It is a vacancy defect (due to missing ions) and also a stoichiometric defect, as the ratio of the number of cations and anions remains the same.
Examples
This type of defect is shown in compounds with :- highly ionic compounds
- high co-ordination number
- small difference in sizes of cations and anions
Examples : NaCl, KCl, CsCl, KBr, AgCl. etc.
Experimental observations show that at room temperature in an NaCl crystal there is one Schottky defect per 1016 ions.

