
Cyclophane
Encyclopedia
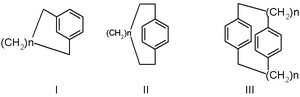
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
consisting of an aromatic unit (typically a benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
ring) and an aliphatic chain that forms a bridge
Bridge
A bridge is a structure built to span physical obstacles such as a body of water, valley, or road, for the purpose of providing passage over the obstacle...
between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cage
Cage (enclosure)
A cage is an enclosure made of mesh, bars or wires, used to confine, contain or protect something or someone. A cage can serve many purposes, including keeping an animal in captivity, capturing, and being used for display of an animal at a zoo.-History:...
like structure
Structure
Structure is a fundamental, tangible or intangible notion referring to the recognition, observation, nature, and permanence of patterns and relationships of entities. This notion may itself be an object, such as a built structure, or an attribute, such as the structure of society...
s are also known. Cyclophanes are well-studied in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
because they adopt unusual chemical conformations due to build-up of
strain
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional...
. Despite this, cyclophane structures are not unknown to biomolecules.
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
and n refers to the number of atoms making up the bridge.
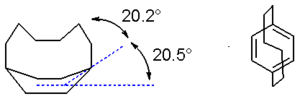
Structure
Paracyclophanes adopt the boat conformation normally observed in cyclohexaneCyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
s but are still able to retain aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
. The smaller the value of n the larger the deviation from aromatic planarity. In [6]paracyclophane which is one of the smallest, yet stable, cyclophanes X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
shows that the aromatic bridgehead carbon atom makes an angle of 20.5° with the plane. The benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
carbons deviate by another 20.2°. The carbon to carbon bond length alternation has increased from 0 for benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
to 39 pm.
In organic reactions [6]cyclophane tends to react as a diene derivative and not as an arene. With bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
it gives 1,4-addition and with chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
the 1,2-addition product forms.
Yet the proton NMR
Proton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
spectrum displays the aromatic protons and their usual deshielded positions around 7.2 ppm and the central methylene protons in the aliphatic bridge are even severely shielded to a position of around - 0.5 ppm, that is, even shielded compared to the internal reference tetramethylsilane
Tetramethylsilane
Tetramethylsilane is the chemical compound with the formula Si4. It is the simplest tetraorganosilane. Like all silanes, the TMS framework is tetrahedral...
. With respect to the diamagnetic ring current criterion for aromaticity this cyclophane is still aromatic.
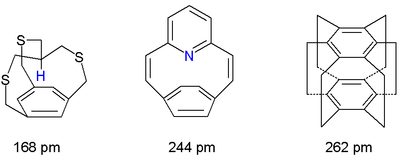
A non-bonding nitrogen to arene distance of 244 pm is recorded for a pyridinophane and in the totally weird superphane
Superphane
[2.2.2.2.2.2]Cyclophane or superphane is a 6-fold bridged cyclophane with all arene positions in the benzene dimer taken up by ethylene spacers. The compound has been of some scientific interest as a model for testing aromaticity and was first synthesised by Boekelheide in 1979. Superphane is the...
the two benzene rings are separated by a mere 262 pm. Another representative of this group are in-methylcyclophane
In-Methylcyclophane
In-Methylcyclophanes are organic compounds and members of a larger family of cyclophanes. These compounds are used to study how chemical bonds in molecules adapt to strain. In-methylcyclophanes in particular have a methyl group in proximity to a benzene ring. This is only possible when both methyl...
s.
Synthetic methods
[6]paracyclophane can be synthesized in the laboratory by a Bamford-Stevens reactionBamford-Stevens reaction
The Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens...
with spiro
Spiro compound
A spiro compound is a bicyclic organic compound with rings connected through just one atom. The rings can be different in nature or identical. The connecting atom is also called the spiroatom, most often a quaternary carbon...
ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
1 in scheme 3 rearranging in a pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
reaction through the carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
intermediate 4. The cyclophane can be photochemically
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
converted to the Dewar benzene
Dewar benzene
Dewar benzene or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1867....
6 and back again by application of heat. A separate route to the Dewar form is by a cationic silver perchlorate
Silver perchlorate
Silver perchlorate is the chemical compound with the formula AgClO4. This white solid forms a monohydrate and is mildly deliquescent. It is a useful source of the Ag+ ion, although the presence of perchlorate presents risks.-Solubility:...
induced rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
of the bicyclopropenyl compound 7.
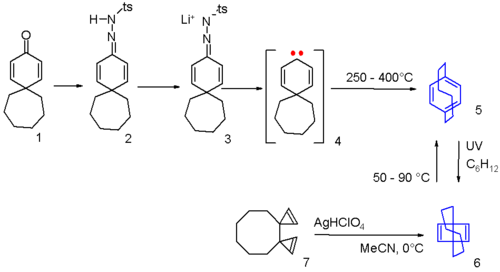
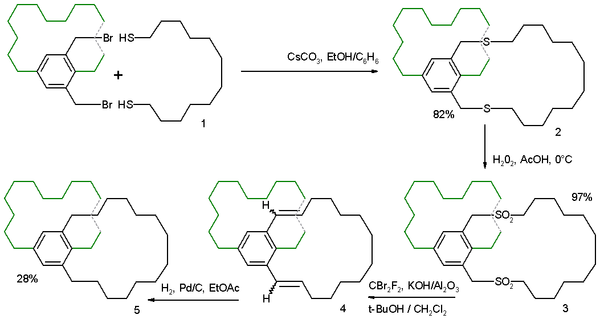
Ramberg-Bäcklund reaction
The Ramberg-Bäcklund Reaction is an organic reaction converting an α-halo sulfone into an alkene in presence of a base with extrusion of sulfur dioxide. The reaction is named after the two Swedish chemists Ludwig Ramberg and Birger Bäcklund. The carbanion formed by deprotonation gives an unstable...
converting the sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
3 to the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
4.
Naturally occurring cyclophanes
Despite carrying strain, the cyclophane motif does exist in nature. One example of a metacyclophane is cavicularinCavicularin
Cavicularin is a natural phenolic secondary metabolite isolated from the liverwort Cavicularia densa. This macrocycle is unusual because it was the first compound isolated from nature displaying optical activity due to the presence of planar chirality and axial chirality. The specific rotation for...
.
Haouamine A is a paracyclophane found in a certain species of tunicate
Tunicate
Tunicates, also known as urochordates, are members of the subphylum Tunicata, previously known as Urochordata, a group of underwater saclike filter feeders with incurrent and excurrent siphons that is classified within the phylum Chordata. While most tunicates live on the ocean floor, others such...
. Because of its potential application as an anticancer drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
it is also available from total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
via an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
- pyrone
Pyrone
Pyrones or pyranones are a class of cyclic chemical compounds. They contain an unsaturated six membered ring containing one oxygen atom and a ketone functional group. There are two isomers denoted as 2-pyrone and 4-pyrone. The 2-pyrone structure is found in nature as part of the coumarin ring...
Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
in the crucial step with expulsion of carbon dioxide (scheme 5).


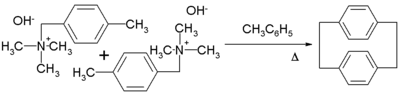
[n,n]Paracyclophanes
A well exploited member of the [n,n]paracyclophane family is [2,2]paracyclophane. One method for its preparation is by a 1,6-Hofmann eliminationHofmann elimination
Hofmann elimination is a process where an amine is reacted to create a tertiary amine and an alkene by treatment with excess methyl iodide followed by treatment with silver oxide, water, and heat.After the first step, a quaternary ammonium iodide salt is created...
:
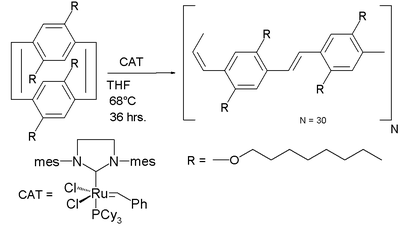
Ring opening metathesis polymerisation
Ring-opening metathesis polymerization is a type of olefin metathesis chain-growth polymerization that produces industrially important products. The driving force of the reaction is relief of ring strain in cyclic olefins and a wide variety of catalysts have been discovered...
to a poly(p-phenylene vinylene)
Poly(p-phenylene vinylene)
Poly is a conducting polymer of the rigid-rod polymer host family.PPV is the only polymer of this type that has so far been successfully processed into a highly ordered crystalline thin film. PPV and its derivatives are conducting polymers of rigid-rod polymer family...
with alternating cis-alkene and trans-alkene bonds using Grubbs' second generation catalyst
Grubbs' catalyst
Grubbs' Catalyst is a transition metal carbene complex named after Robert H. Grubbs, the chemist who first synthesized it. There are two generations of the catalyst, as shown on the right. In contrast to other olefin metathesis catalysts, Grubbs' Catalysts tolerate other functional groups in the...
:
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
due to the lack of competing reactions.
Because the two benzene rings are in close proximity this cyclophane type also serves as guinea pig for photochemical dimerization reactions as illustrated by this example:
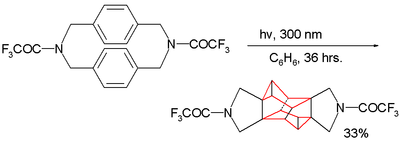
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group is replaced by a methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
group no reaction takes place: the dimerization requires through-bond overlap between the aromatic pi electrons and the sigma electrons
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
in the C-N bond in the reactants LUMO
Lumo
Lumo is a 2007 documentary film about twenty-year-old Lumo Sinai, a woman who fell victim to "Africa's First World War." While returning home one day, Lumo and another woman were gang-raped by a group of soldiers fighting for control of the Democratic Republic of the Congo during the 1994 Rwandan...
.

