Hofmann elimination
Encyclopedia
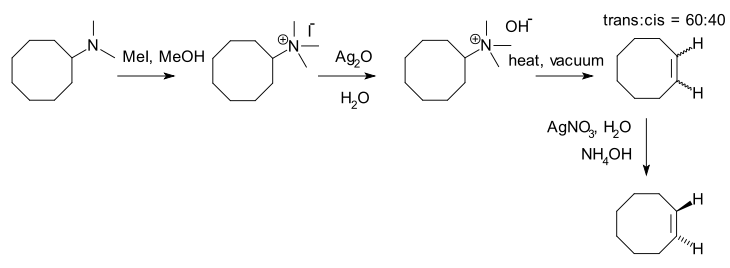
Hofmann elimination is a process where an amine
is reacted to create a tertiary amine and an alkene
by treatment with excess methyl iodide followed by treatment with silver oxide, water
, and heat.
After the first step, a quaternary ammonium iodide
salt is created. After replacement of iodine by an hydroxyl
anion, an elimination reaction
takes place to the alkene.
With unsymmetrical amines, the major alkene product is the least substituted and generally the least stable, an observation known as the Hofmann rule. This is in direct contrast to normal elimination reactions where the more substituted, stable product is dominant (Zaitsev's rule
).
The reaction is named after its discoverer: August Wilhelm von Hofmann
.
An example is the synthesis of trans-cyclooctene :
In a related chemical test
called Herzig-Meyer alkimide group determination a tertiary amine with at least one methyl group and lacking a beta-proton is allowed to react with hydrogen iodide
to the quaternary ammonium salt which when heated degrades to iodomethane
and the secondary amine.
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
is reacted to create a tertiary amine and an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
by treatment with excess methyl iodide followed by treatment with silver oxide, water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, and heat.
After the first step, a quaternary ammonium iodide
Ammonium iodide
Ammonium iodide is the chemical compound NH4I. It is used in photographic chemicals and some medications. It can be prepared by the action of hydroiodic acid on ammonia. It is easily soluble in water, from which it crystallizes in cubes. It is also soluble in ethanol...
salt is created. After replacement of iodine by an hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
anion, an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
takes place to the alkene.
With unsymmetrical amines, the major alkene product is the least substituted and generally the least stable, an observation known as the Hofmann rule. This is in direct contrast to normal elimination reactions where the more substituted, stable product is dominant (Zaitsev's rule
Zaitsev's rule
In chemistry, Zaitsev's rule, Saytzeff's rule or Saytsev's rule named after Alexander Mikhailovich Zaitsev is a rule that states that if more than one alkene can be formed during dehalogenation by an elimination reaction, the more stable alkene is the major product...
).
The reaction is named after its discoverer: August Wilhelm von Hofmann
August Wilhelm von Hofmann
August Wilhelm von Hofmann was a German chemist.-Biography:Hofmann was born at Gießen, Grand Duchy of Hesse. Not intending originally to devote himself to physical science, he first took up the study of law and philology at Göttingen. But he then turned to chemistry, and studied under Justus von...
.
An example is the synthesis of trans-cyclooctene :
In a related chemical test
Chemical test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to prove the existence of, or to quantify, a chemical compound or chemical group with the aid of a specific reagent...
called Herzig-Meyer alkimide group determination a tertiary amine with at least one methyl group and lacking a beta-proton is allowed to react with hydrogen iodide
Hydrogen iodide
Hydrogen iodide is a diatomic molecule. Aqueous solutions of HI are known as iohydroic acid or hydroiodic acid, a strong acid. Gas and aqueous solution are interconvertible...
to the quaternary ammonium salt which when heated degrades to iodomethane
Iodomethane
Methyl iodide, also called iodomethane, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally...
and the secondary amine.