Cyclen
Encyclopedia
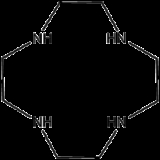
Cyclen or 1,4,7,10-tetraazacyclododecane is a macrocycle
and the aza
analogue of the crown ether
12-crown-4
. Derivatives of cyclen are larger cyclic polyamine
s but the repeating unit (ethyleneimine) is always the same. Like crown ethers, cyclen compounds are capable of selectively binding cations. They are used as a ligand
in chemistry
for instance with chemicals used in MRI contrast agents.
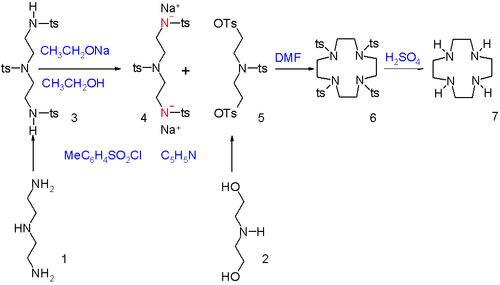
groups in diethylene triamine
(1) are activated as amine anionic nucleophiles by reaction with tosyl chloride in pyridine
to the N-tosyl protective group followed by proton abstraction with sodium ethoxide
. The alcohol
end groups in diethanolamine (2) are activated as electrophile
by converting them into tosyl leaving group
s. The two segments are joined in dimethylformamide
and unless the reactants are very diluted, ordinary polymerization
will take place to long linear chains and not cyclization. In the final step the tosyl groups are removed with sulfuric acid
.
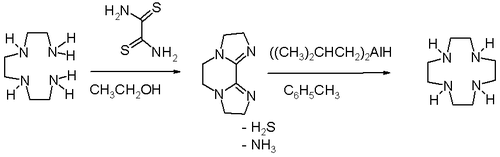
High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide
to a bisamidine
(also a bis(imidazoline
)) followed by reduction and ring expansion with DIBAL.
In one study cyclen is covalently
bonded through a propylene
molecular spacer
to adenine
and chelated
with zinc
diperchlorate
. This complex is able to selectively bind uracil
and uridine
in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evidenced by mass spectrometry
.
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
and the aza
Aza-
The prefix aza- is used in organic chemistry to form names of organic compounds where a carbon atom is replaced by a nitrogen atom. Sometimes a number between hyphens is inserted before it to state which atom the nitrogen atom replaces...
analogue of the crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
12-crown-4
12-Crown-4
12-crown-4, also called 1,4,7,10-tetraoxacyclododecane and Lithium Ionophore V, is a crown ether with the formula C8H16O4. It is a cyclic tetramer of ethylene oxide which is specific for the lithium cation:-See also:*Crown ether...
. Derivatives of cyclen are larger cyclic polyamine
Polyamine
A polyamine is an organic compound having two or more primary amino groups .This class of compounds includes several synthetic substances that are important feedstocks for the chemical industry, such as ethylene diamine , 1,3-diaminopropane , and hexamethylenediamine...
s but the repeating unit (ethyleneimine) is always the same. Like crown ethers, cyclen compounds are capable of selectively binding cations. They are used as a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
for instance with chemicals used in MRI contrast agents.
Synthesis
Cyclen compounds can be synthesized by combining two separate parts by nucleophilic displacement. In this procedure the terminal amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
groups in diethylene triamine
Diethylene triamine
Diethylenetriamine is an organic compound with the formula HN2. This colourless hygroscopic liquid is soluble in water and polar organic solvents, but not simple hydrocarbons. Diethylenetriamine is structural analogue of diethylene glycol. Its chemical properties resemble those for ethylene...
(1) are activated as amine anionic nucleophiles by reaction with tosyl chloride in pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
to the N-tosyl protective group followed by proton abstraction with sodium ethoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
. The alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
end groups in diethanolamine (2) are activated as electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
by converting them into tosyl leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
s. The two segments are joined in dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
and unless the reactants are very diluted, ordinary polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
will take place to long linear chains and not cyclization. In the final step the tosyl groups are removed with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
.
High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide
Dithiooxamide
Dithiooxamide or rubeanic acid is an organic compound. It is the sulfur analog of oxamide. It acts as a chelating agent, e.g. in the detection or determination of copper. It has also been used as a building block in the synthesis of cyclen....
to a bisamidine
Amidine
Amidines are a class of oxoacid derivatives.The oxoacid from which an amidine is derived must be of the form RnEOH, where R is a substituent...
(also a bis(imidazoline
Imidazoline
Imidazoline is a nitrogen-containing heterocycle with formula C3H6N2, derived from imidazole. The ring contains an imine bond, and the carbons at the 4 and 5 positions are singly bonded, rather than doubly bonded for the case of imidazole...
)) followed by reduction and ring expansion with DIBAL.
In one study cyclen is covalently
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
bonded through a propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
molecular spacer
Molecular spacer
A Molecular spacer or simply a spacer in chemistry is any flexible part of a molecule providing a connection betweentwo other parts of a molecule....
to adenine
Adenine
Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
and chelated
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
diperchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
. This complex is able to selectively bind uracil
Uracil
Uracil is one of the four nucleobases in the nucleic acid of RNA that are represented by the letters A, G, C and U. The others are adenine, cytosine, and guanine. In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine.Uracil is a common and...
and uridine
Uridine
Uridine is a molecule that is formed when uracil is attached to a ribose ring via a β-N1-glycosidic bond.If uracil is attached to a deoxyribose ring, it is known as a deoxyuridine....
in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evidenced by mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
.