
Amidine
Encyclopedia
Amidines are a class of oxoacid
derivatives
.
The oxoacid from which an amidine is derived must be of the form RnE(=O)OH, where R is a substituent
. The −OH group
is replaced by an −NH2 group
and the =O
group is replaced by =N
R
, giving amidines the general structure RnE(=NR)NR2 .
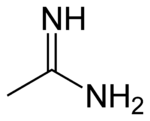 When the parent oxoacid is a carboxylic acid
When the parent oxoacid is a carboxylic acid
, the resulting amidine is a carboxamidine or carboximidamide (IUPAC name), and has the following general structure:
 Carboxamidines are frequently referred to simply as amidines, as they are the most commonly encountered type of amidine in organic chemistry
Carboxamidines are frequently referred to simply as amidines, as they are the most commonly encountered type of amidine in organic chemistry
. The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines include DBU and diminazene
.
The most common way to make primary amidines is by the Pinner reaction
.
with an organometallic compound such as methyl lithium
. They are used widely as ligands in organometallic complexes.
Oxoacid
An oxoacid is an acid that contains oxygen. To be more specific, it is an acid that:#contains oxygen#contains at least one other element#has at least one hydrogen atom bound to oxygen#forms an ion by the loss of one or more protons....
derivatives
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by some chemical or physical process. In the past it was also used to mean a compound that can be imagined to arise from another compound, if one atom is replaced with another atom or group of atoms, but modern...
.
The oxoacid from which an amidine is derived must be of the form RnE(=O)OH, where R is a substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
. The −OH group
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
is replaced by an −NH2 group
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
and the =O
Oxo
OXO was the first digital graphical computer game, a version of Tic-tac-toe.It is also the first puzzler game; As seen on Ginuess World Records 2010 Gamer's Edition.OXO Was first released in 1951, That makes it one of the oldest games standing....
group is replaced by =N
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
R
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
, giving amidines the general structure RnE(=NR)NR2 .
Carboxamidines

Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
, the resulting amidine is a carboxamidine or carboximidamide (IUPAC name), and has the following general structure:

Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines include DBU and diminazene
Diminazene
Diminazen is a di-amidine also known as 4,4'-bis. It binds DNA and RNA and is the acting component of diminazene aceturat drugs directed, e.g., against trypanosomiasis....
.
The most common way to make primary amidines is by the Pinner reaction
Pinner reaction
The Pinner reaction is an organic reaction of a nitrile with an alcohol under acid catalysis for instance hydrochloric acid. The product formed is the hydrochloric acid salt of an imino ester or an alkyl imidate, which sometimes is called a Pinner salt...
.
Amidinate salts
An amidinate salt has the general structure M+[RNRCNR]- and can be accessed by reaction of a carbodiimideCarbodiimide
A carbodiimide or a methanediimine is a functional group consisting of the formula RN=C=NR. Carbodiimides hydrolyze to form ureas, which makes them uncommon in nature.-Carbodiimide formation:...
with an organometallic compound such as methyl lithium
Methyl lithium
Methyllithium is an organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used as a solution in ethers, is a reagent in organic synthesis as well...
. They are used widely as ligands in organometallic complexes.
See Also
- Guanidines - a similar group of compounds where the central Carbon is bonded to three Nitrogens.

