
Barium
Encyclopedia
Barium is a chemical element
with the symbol Ba and atomic number
56. It is the fifth element in Group 2, a soft silvery metal
lic alkaline earth metal
. Barium is never found in nature in its pure form due to its reactivity with air
. Its oxide is historically known as baryta
but it reacts with water and carbon dioxide and is not found as a mineral. The most common naturally occurring minerals are the very insoluble barium sulfate, BaSO4 (barite
), and barium carbonate
, BaCO3 (witherite
). Barium's name originates from Greek
barys (βαρύς), meaning "heavy", describing the high density of some common barium-containing ores.
Barium has few industrial applications, but the metal has been historically used to scavenge air
in vacuum tube
s. Barium compounds impart a green color to flames and have been used in fireworks. Barium sulfate
is used for its density, insolubility, and X-ray opacity. It is used as an insoluble heavy mud-like paste when drilling oil wells, and in purer form, as an X-ray radiocontrast agent for imaging the human gastrointestinal tract. Soluble barium compounds are poisonous due to release of the soluble barium ion, and have been used as rodenticides. New uses for barium continue to be sought. It is a component of some "high temperature" YBCO superconductors, and electroceramics.
, resulting from emission at 524.2 and 513.7 nm. Its simple compounds are notable for their relatively high (for an alkaline earth element) specific gravity
. This high density is true of the most common barium-bearing mineral, barite
(BaSO4), also called 'heavy spar' due to the high density (4.5 g/cm³).
and peroxide
. Because of its sensitivity to air, samples are generally stored under protective oils. The reaction is violent if barium is powdered. The metal is readily attacked in most acids, with the notable exception of sulfuric acid
, as passivation
stops the reaction by forming the insoluble barium sulfate
. It also reacts violently with water according to the reaction:
Barium combines with several metals, including aluminium, zinc, lead and tin, forming intermetallic phases
and alloys.
s, the most abundant being 138Ba (71.7 %). 22 isotopes are known, but most of these are highly radioactive and have half-lives
in the several millisecond to several day range. The only notable exceptions are 133Ba which has a half-life of 10.51 years, and 137mBa (2.55 minutes). 133Ba is a standard calibrant for gamma-ray detectors in nuclear physics studies.
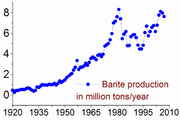
, today nearly all barium is mined as barite.
Large deposits of barite are found in China
, Germany
, India
, Morocco
, and in the United States
.
A rare gem containing barium is known, called benitoite
.
 Because barium quickly oxidizes in air, it is difficult to obtain the free metal and it is never found free in nature. The metal is primarily found in, and extracted from, barite. Because barite is so insoluble, it cannot be used directly for the preparation of other barium compounds, or barium metal. Instead, the ore is heated with carbon
Because barium quickly oxidizes in air, it is difficult to obtain the free metal and it is never found free in nature. The metal is primarily found in, and extracted from, barite. Because barite is so insoluble, it cannot be used directly for the preparation of other barium compounds, or barium metal. Instead, the ore is heated with carbon
to reduce it to barium sulfide
:
The barium sulfide is then hydrolyzed
or treated with acids to form other barium compounds, such as the chloride
, nitrate
, and carbonate.
Barium is commercially produced through the electrolysis
of molten barium chloride (BaCl2):
Ba2+ + 2 → Ba 2 Cl– → Cl2 + 2
Barium metal is also obtained by the reduction of barium oxide with finely divided aluminium
at temperatures between 1100 and 1200 °C:
The barium vapor is cooled and condensed to give the solid metal, which can be cast into rods or extruded into wires. Being a flammable solid, it is packaged under argon
in steel containers or plastic bags.
throughout the chemistry of barium. Its properties generally resemble those of other alkaline earth ions such as strontium and calcium. All halides, pseudohalides and chalcogenides are known, usually as colourless solids. The sulfate is famously insoluble. BaO forms a peroxide when heated in air. The oxide is basic and reacts with acids to give salts.
Barium reduces oxides, chlorides and sulfides of less active metals. For example:
At elevated temperatures, barium combines with nitrogen
and hydrogen
to produce the nitride
Ba3N2 and hydride
BaH2, respectively. When heated with nitrogen and carbon, it forms the cyanide:
, Italy
were known as "Bologna stones". Witches and alchemists were attracted to them because after exposure to light they would glow for years.
Carl Scheele identified barite as containing a new element in 1774, but could not isolate barium, only barium oxide
. Johan Gottlieb Gahn
also isolated barium oxide
two years later in similar studies. Oxidized barium was at first called barote, by Guyton de Morveau, a name which was changed by Antoine Lavoisier
to baryta. Also in the 18th century, English mineralogist William Withering
noted a heavy mineral in the lead mines of cumberland
, now known to be Witherite
. Barium was first isolated by electrolysis of molten barium salts in 1808, by Sir Humphry Davy
in England
. Davy, by analogy with calcium
named "barium" after baryta, with the "-ium" ending signifying a metallic element. Robert Bunsen
and Augustus Matthiessen
yielded pure barium by electrolysis of a molten mixture of barium chloride and ammonium chloride.
The production of pure oxygen in the Brin process
was a large scale application of barium peroxide before electrolysis and fractionally distill
liquefied air became the dominant ways to produce oxygen. In this process the barium oxide reacts at 500–600°C with air to form barium peroxide which decomposes at above 700°C by releasing oxygen.
 The dominating application of elemental barium is as a scavenger or "getter
The dominating application of elemental barium is as a scavenger or "getter
" removing the last traces of oxygen and other gases in electronic vacuum tubes such as television cathode ray tubes.
An alloy of barium with nickel
is commonly used in automobile ignitions.
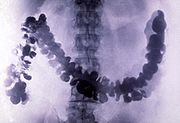
s. It is also a filler in a variety of products such as rubber. Taking advantage of its opacity to X-rays, the sulfate is used as a radiocontrast
agent for X-ray imaging
of the digestive system ("barium meal
s" and "barium enemas"). Lithopone
, a pigment
that contains barium sulfate and zinc sulfide
, is a permanent white that has good covering power, and does not darken when exposed to sulfides.
), which is not a problem for the insoluble BaSO4.
ous. At low doses, barium acts as a muscle stimulant, whereas higher doses affect the nervous system
, causing cardiac irregularities, tremors, weakness
, anxiety
, dyspnea
and paralysis
. This may be due to its ability to block potassium ion channels which are critical to the proper function of the nervous system. However, individual responses to barium salts vary widely, with some being able to handle barium nitrate casually without problems, and others becoming ill from working with it in small quantities. For example, barium acetate
was used by Marie Robards to poison her father in 1993.
ly. It is eliminated completely from the digestive tract. Unlike other heavy metals
, barium does not bioaccumulate
. However, inhaled dust containing barium compounds can accumulate in the lungs, causing a benign
condition called baritosis
.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with the symbol Ba and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
56. It is the fifth element in Group 2, a soft silvery metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
lic alkaline earth metal
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
. Barium is never found in nature in its pure form due to its reactivity with air
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
. Its oxide is historically known as baryta
Barium hydroxide
Barium hydroxide is the chemical compound with the formula Ba2. Also known as baryta, it is one of the principal compounds of barium. The white granular monohydrate is the usual commercial form.-Preparation:...
but it reacts with water and carbon dioxide and is not found as a mineral. The most common naturally occurring minerals are the very insoluble barium sulfate, BaSO4 (barite
Barite
Baryte, or barite, is a mineral consisting of barium sulfate. The baryte group consists of baryte, celestine, anglesite and anhydrite. Baryte itself is generally white or colorless, and is the main source of barium...
), and barium carbonate
Barium carbonate
Barium carbonate , also known as witherite, is a chemical compound used in rat poison, bricks, ceramic glazes and cement.Witherite crystallizes in the orthorhombic system...
, BaCO3 (witherite
Witherite
Witherite is a barium carbonate mineral, BaCO3, in the aragonite group. Witherite crystallizes in the orthorhombic system and virtually always is twinned. The mineral is colorless, milky white, grey, pale yellow, green, to pale brown. The specific gravity is 4.3, which is high for a translucent...
). Barium's name originates from Greek
Greek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
barys (βαρύς), meaning "heavy", describing the high density of some common barium-containing ores.
Barium has few industrial applications, but the metal has been historically used to scavenge air
Getter
A getter is a deposit of reactive material that is deliberately placed inside a vacuum system, for the purpose of completing and maintaining the vacuum. When gas molecules strike the getter material, they combine with it chemically or by adsorption...
in vacuum tube
Vacuum tube
In electronics, a vacuum tube, electron tube , or thermionic valve , reduced to simply "tube" or "valve" in everyday parlance, is a device that relies on the flow of electric current through a vacuum...
s. Barium compounds impart a green color to flames and have been used in fireworks. Barium sulfate
Barium sulfate
Barium sulfate is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it...
is used for its density, insolubility, and X-ray opacity. It is used as an insoluble heavy mud-like paste when drilling oil wells, and in purer form, as an X-ray radiocontrast agent for imaging the human gastrointestinal tract. Soluble barium compounds are poisonous due to release of the soluble barium ion, and have been used as rodenticides. New uses for barium continue to be sought. It is a component of some "high temperature" YBCO superconductors, and electroceramics.
Physical properties
Barium is a soft, silvery white alkali earth metal, which quickly oxidizes in air. It crystallizes in body centered cubic lattices. It burns with a green to pale green flameFlame test
A flame test is a procedure used in chemistry to detect the presence of certain metal ions, based on each element's characteristic emission spectrum. The color of flames in general also depends on temperature; see flame color....
, resulting from emission at 524.2 and 513.7 nm. Its simple compounds are notable for their relatively high (for an alkaline earth element) specific gravity
Specific gravity
Specific gravity is the ratio of the density of a substance to the density of a reference substance. Apparent specific gravity is the ratio of the weight of a volume of the substance to the weight of an equal volume of the reference substance. The reference substance is nearly always water for...
. This high density is true of the most common barium-bearing mineral, barite
Barite
Baryte, or barite, is a mineral consisting of barium sulfate. The baryte group consists of baryte, celestine, anglesite and anhydrite. Baryte itself is generally white or colorless, and is the main source of barium...
(BaSO4), also called 'heavy spar' due to the high density (4.5 g/cm³).
Chemical properties
Barium, as for other alkali earth (group II) metals, is highly reducing. It reacts exothermically with oxygen at room temperature to form barium oxideBarium oxide
Barium oxide, BaO, is a white hygroscopic compound formed by the burning of barium in oxygen, although it is often formed through the decomposition of other barium salts.It reacts with water to form barium hydroxide.-Uses:...
and peroxide
Barium peroxide
Barium peroxide is the chemical compound with the formula BaO2. This grey-white solid is one of the most common inorganic peroxides. Barium peroxide is an oxidizing agent, which is used for bleaching...
. Because of its sensitivity to air, samples are generally stored under protective oils. The reaction is violent if barium is powdered. The metal is readily attacked in most acids, with the notable exception of sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, as passivation
Passivation
Passivation is the process of making a material "passive", and thus less reactive with surrounding air, water, or other gases or liquids. The goal is to inhibit corrosion, whether for structural or cosmetic reasons. Passivation of metals is usually achieved by the deposition of a layer of oxide...
stops the reaction by forming the insoluble barium sulfate
Barium sulfate
Barium sulfate is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it...
. It also reacts violently with water according to the reaction:
- Ba + 2 H2O → Ba(OH)2 + H2↑
Barium combines with several metals, including aluminium, zinc, lead and tin, forming intermetallic phases
Intermetallics
Intermetallics or intermetallic compounds is a term that is used in a number of different ways. Most commonly it refers to solid-state phases involving metals. There is a "research definition" adhered to generally in scientific publications, and a wider "common use" term...
and alloys.
Isotopes
Naturally occurring barium is a mix of seven stable isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s, the most abundant being 138Ba (71.7 %). 22 isotopes are known, but most of these are highly radioactive and have half-lives
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
in the several millisecond to several day range. The only notable exceptions are 133Ba which has a half-life of 10.51 years, and 137mBa (2.55 minutes). 133Ba is a standard calibrant for gamma-ray detectors in nuclear physics studies.
Occurrence
The abundance of barium is 0.0425 % in the Earth's crust and 13 µg/L in sea water. It occurs in the minerals barite (as the sulfate) and witherite (as the carbonate). Although witherite deposits were mined from the 17th century till 1969 in northern England, for example in the Settlingstones Mine near NewbroughNewbrough
Newbrough is a village in Northumberland, England. The village lies on the north bank of the River South Tyne about west of Hexham.- History :...
, today nearly all barium is mined as barite.
Large deposits of barite are found in China
China
Chinese civilization may refer to:* China for more general discussion of the country.* Chinese culture* Greater China, the transnational community of ethnic Chinese.* History of China* Sinosphere, the area historically affected by Chinese culture...
, Germany
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
, India
India
India , officially the Republic of India , is a country in South Asia. It is the seventh-largest country by geographical area, the second-most populous country with over 1.2 billion people, and the most populous democracy in the world...
, Morocco
Morocco
Morocco , officially the Kingdom of Morocco , is a country located in North Africa. It has a population of more than 32 million and an area of 710,850 km², and also primarily administers the disputed region of the Western Sahara...
, and in the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
.
A rare gem containing barium is known, called benitoite
Benitoite
Benitoite is a rare blue barium titanium silicate mineral, found in hydrothermally altered serpentinite. Benitoite fluoresces under short wave ultraviolet light, appearing bright blue to bluish white in color. The more rarely seen clear to white benitoite crystals fluoresce red under long-wave UV...
.
Production

Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
to reduce it to barium sulfide
Barium sulfide
Barium sulfide is the inorganic compound with the formula BaS. BaS is an important precursor to other barium compounds including BaCO3 and the pigment lithopone, ZnS/BaSO4. Like other chalcogenides of the alkaline earth metals, BaS is a short wavelength emitters for electronic displays...
:
- BaSO4 + 2 C → BaS + 2 CO2
The barium sulfide is then hydrolyzed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
or treated with acids to form other barium compounds, such as the chloride
Barium chloride
Barium chloride is the inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like other barium salts, it is toxic and imparts a yellow-green coloration to a flame. It is also hygroscopic....
, nitrate
Barium nitrate
Barium nitrate with chemical formula Ba2 is a salt of barium and the nitrate ion.Barium nitrate exists as a white solid at room temperature. It is soluble in water, and like other soluble barium compounds, is toxic and should be handled with care...
, and carbonate.
Barium is commercially produced through the electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of molten barium chloride (BaCl2):
Ba2+ + 2 → Ba 2 Cl– → Cl2 + 2
Barium metal is also obtained by the reduction of barium oxide with finely divided aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
at temperatures between 1100 and 1200 °C:
- 4 BaO + 2 Al → BaO·Al2O3 + 3 Ba
The barium vapor is cooled and condensed to give the solid metal, which can be cast into rods or extruded into wires. Being a flammable solid, it is packaged under argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
in steel containers or plastic bags.
Compounds
Ba2+ is the dominant oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
throughout the chemistry of barium. Its properties generally resemble those of other alkaline earth ions such as strontium and calcium. All halides, pseudohalides and chalcogenides are known, usually as colourless solids. The sulfate is famously insoluble. BaO forms a peroxide when heated in air. The oxide is basic and reacts with acids to give salts.
Barium reduces oxides, chlorides and sulfides of less active metals. For example:
- Ba + CdO → BaO + Cd
- Ba + ZnCl2 → BaCl2 + Zn
- 3 Ba + Al2S3 → 3 BaS + 2 Al
At elevated temperatures, barium combines with nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
to produce the nitride
Nitride
In chemistry, a nitride is a compound of nitrogen where nitrogen has a formal oxidation state of −3. Nitrides are a large class of compounds with a wide range of properties and applications....
Ba3N2 and hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
BaH2, respectively. When heated with nitrogen and carbon, it forms the cyanide:
- Ba + N2 + 2 C → Ba(CN)2
History
Barium's name originates from Greek barys, meaning "heavy", describing the density of some common barium-containing ores. Alchemists in the early Middle Ages knew about some barium minerals. Smooth pebble-like stones of mineral barite found in BolognaBologna
Bologna is the capital city of Emilia-Romagna, in the Po Valley of Northern Italy. The city lies between the Po River and the Apennine Mountains, more specifically, between the Reno River and the Savena River. Bologna is a lively and cosmopolitan Italian college city, with spectacular history,...
, Italy
Italy
Italy , officially the Italian Republic languages]] under the European Charter for Regional or Minority Languages. In each of these, Italy's official name is as follows:;;;;;;;;), is a unitary parliamentary republic in South-Central Europe. To the north it borders France, Switzerland, Austria and...
were known as "Bologna stones". Witches and alchemists were attracted to them because after exposure to light they would glow for years.
Carl Scheele identified barite as containing a new element in 1774, but could not isolate barium, only barium oxide
Barium oxide
Barium oxide, BaO, is a white hygroscopic compound formed by the burning of barium in oxygen, although it is often formed through the decomposition of other barium salts.It reacts with water to form barium hydroxide.-Uses:...
. Johan Gottlieb Gahn
Johan Gottlieb Gahn
Johan Gottlieb Gahn was a Swedish chemist and metallurgist who discovered manganese in 1774.Gahn studied in Uppsala 1762-1770 and became acquainted with chemists Torbern Bergman och Carl Wilhelm Scheele...
also isolated barium oxide
Barium oxide
Barium oxide, BaO, is a white hygroscopic compound formed by the burning of barium in oxygen, although it is often formed through the decomposition of other barium salts.It reacts with water to form barium hydroxide.-Uses:...
two years later in similar studies. Oxidized barium was at first called barote, by Guyton de Morveau, a name which was changed by Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
to baryta. Also in the 18th century, English mineralogist William Withering
William Withering
William Withering was an English botanist, geologist, chemist, physician and the discoverer of digitalis.-Introduction:...
noted a heavy mineral in the lead mines of cumberland
Cumberland
Cumberland is a historic county of North West England, on the border with Scotland, from the 12th century until 1974. It formed an administrative county from 1889 to 1974 and now forms part of Cumbria....
, now known to be Witherite
Witherite
Witherite is a barium carbonate mineral, BaCO3, in the aragonite group. Witherite crystallizes in the orthorhombic system and virtually always is twinned. The mineral is colorless, milky white, grey, pale yellow, green, to pale brown. The specific gravity is 4.3, which is high for a translucent...
. Barium was first isolated by electrolysis of molten barium salts in 1808, by Sir Humphry Davy
Humphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
in England
England
England is a country that is part of the United Kingdom. It shares land borders with Scotland to the north and Wales to the west; the Irish Sea is to the north west, the Celtic Sea to the south west, with the North Sea to the east and the English Channel to the south separating it from continental...
. Davy, by analogy with calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
named "barium" after baryta, with the "-ium" ending signifying a metallic element. Robert Bunsen
Robert Bunsen
Robert Wilhelm Eberhard Bunsen was a German chemist. He investigated emission spectra of heated elements, and discovered caesium and rubidium with Gustav Kirchhoff. Bunsen developed several gas-analytical methods, was a pioneer in photochemistry, and did early work in the field of organoarsenic...
and Augustus Matthiessen
Augustus Matthiessen
Augustus Matthiessen, FRS , the son of a merchant, was a British chemist and physicist who obtained his PhD in Germany at the University of Gießen in 1852 with Johann Heinrich Buff. He then worked with Robert Bunsen at the University of Heidelberg from 1853 to 1856...
yielded pure barium by electrolysis of a molten mixture of barium chloride and ammonium chloride.
The production of pure oxygen in the Brin process
Brin process
Brin process is a now obsolete industrial scale production process for oxygen. In this process the barium oxide reacts at 500–600°C with air to form barium peroxide which decomposes at above 800°C by releasing oxygen....
was a large scale application of barium peroxide before electrolysis and fractionally distill
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
liquefied air became the dominant ways to produce oxygen. In this process the barium oxide reacts at 500–600°C with air to form barium peroxide which decomposes at above 700°C by releasing oxygen.
- 2 BaO + O2 ⇌ 2 BaO2
Applications


Getter
A getter is a deposit of reactive material that is deliberately placed inside a vacuum system, for the purpose of completing and maintaining the vacuum. When gas molecules strike the getter material, they combine with it chemically or by adsorption...
" removing the last traces of oxygen and other gases in electronic vacuum tubes such as television cathode ray tubes.
An alloy of barium with nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
is commonly used in automobile ignitions.
Applications of barium sulfate
Barium sulfate (the mineral barite, BaSO4) is important to the petroleum industry, for example, as drilling mud, a weighting agent in drilling new oil wellOil well
An oil well is a general term for any boring through the earth's surface that is designed to find and acquire petroleum oil hydrocarbons. Usually some natural gas is produced along with the oil. A well that is designed to produce mainly or only gas may be termed a gas well.-History:The earliest...
s. It is also a filler in a variety of products such as rubber. Taking advantage of its opacity to X-rays, the sulfate is used as a radiocontrast
Radiocontrast
Radiocontrast agents are a type of medical contrast medium used to improve the visibility of internal bodily structures in an X-ray based imaging techniques such as computed tomography or radiography...
agent for X-ray imaging
Medical imaging
Medical imaging is the technique and process used to create images of the human body for clinical purposes or medical science...
of the digestive system ("barium meal
Barium meal
A barium meal, also known as an upper gastrointestinal series is a procedure in which radiographs of the esophagus, stomach and duodenum are taken after barium sulfate is ingested by a patient...
s" and "barium enemas"). Lithopone
Lithopone
Lithophone is a white pigment consisting of a mixture of barium sulfate and zinc sulfide. It is used in interior paints and in some enamels. It is widely used for white paint.-History:...
, a pigment
Pigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
that contains barium sulfate and zinc sulfide
Zinc sulfide
Zinc sulfide is a inorganic compound with the formula ZnS. ZnS is the main form of zinc in nature, where it mainly occurs as the mineral sphalerite...
, is a permanent white that has good covering power, and does not darken when exposed to sulfides.
Applications of other barium compounds
Aside from the sulfate, other compounds of barium find only niche applications. Applications are limited by the toxicity of Ba2+ ions (Barium carbonate is a rat poisonRat poison
Rodenticides are a category of pest control chemicals intended to kill rodents.Single feed baits are chemicals sufficiently dangerous that the first dose is sufficient to kill....
), which is not a problem for the insoluble BaSO4.
- Barium oxideBarium oxideBarium oxide, BaO, is a white hygroscopic compound formed by the burning of barium in oxygen, although it is often formed through the decomposition of other barium salts.It reacts with water to form barium hydroxide.-Uses:...
is used in a coating for the electrodeElectrodeAn electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s of fluorescent lampFluorescent lampA fluorescent lamp or fluorescent tube is a gas-discharge lamp that uses electricity to excite mercury vapor. The excited mercury atoms produce short-wave ultraviolet light that then causes a phosphor to fluoresce, producing visible light. A fluorescent lamp converts electrical power into useful...
s, which facilitates the release of electronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s. - Barium carbonateBarium carbonateBarium carbonate , also known as witherite, is a chemical compound used in rat poison, bricks, ceramic glazes and cement.Witherite crystallizes in the orthorhombic system...
is also used in glassGlassGlass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
making. Being a heavy element, barium increases the refractive indexRefractive indexIn optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
and luster of the glass. - Barium, commonly as barium nitrateBarium nitrateBarium nitrate with chemical formula Ba2 is a salt of barium and the nitrate ion.Barium nitrate exists as a white solid at room temperature. It is soluble in water, and like other soluble barium compounds, is toxic and should be handled with care...
, is used to give green colors in fireworks. The species responsible for the brilliant green is barium monochloride; in the absence of a source of chlorine a yellow or "apple" green is produced instead. - Barium peroxideBarium peroxideBarium peroxide is the chemical compound with the formula BaO2. This grey-white solid is one of the most common inorganic peroxides. Barium peroxide is an oxidizing agent, which is used for bleaching...
can be used as a catalyst to start an aluminothermic reactionAluminothermic reactionAluminothermic reactions are exothermic chemical reactions using aluminium as the reducing agent at high temperature. The most prominent example is the thermite reaction between aluminium and iron oxides:-History:...
when welding rail tracks together. It can also be used in green tracer ammunitionTracer ammunitionTracer ammunition are bullets that are built with a small pyrotechnic charge in their base. Ignited by the burning powder, the phosphorus tail burns very brightly, making the projectile visible to the naked eye...
and as a bleaching agent. - Barium titanateBarium titanateBarium titanate is the inorganic compound with the chemical formula BaTiO3. Barium titanate is a white powder and transparent as larger crystals...
is a promising electroceramic. - Barium fluorideBarium fluorideBarium fluoride is a chemical compound of barium and fluorine. It is a solid which can be a transparent crystal. It occurs in nature as the mineral frankdicksonite.-Structure:...
is used for optics in infrared applications, since it is transparent from about 0.15 to 12 micrometres.
Precautions
Soluble barium compounds are poisonPoison
In the context of biology, poisons are substances that can cause disturbances to organisms, usually by chemical reaction or other activity on the molecular scale, when a sufficient quantity is absorbed by an organism....
ous. At low doses, barium acts as a muscle stimulant, whereas higher doses affect the nervous system
Nervous system
The nervous system is an organ system containing a network of specialized cells called neurons that coordinate the actions of an animal and transmit signals between different parts of its body. In most animals the nervous system consists of two parts, central and peripheral. The central nervous...
, causing cardiac irregularities, tremors, weakness
Muscle weakness
Muscle weakness or myasthenia is a lack of muscle strength. The causes are many and can be divided into conditions that have true or perceived muscle weakness...
, anxiety
Anxiety
Anxiety is a psychological and physiological state characterized by somatic, emotional, cognitive, and behavioral components. The root meaning of the word anxiety is 'to vex or trouble'; in either presence or absence of psychological stress, anxiety can create feelings of fear, worry, uneasiness,...
, dyspnea
Dyspnea
Dyspnea , shortness of breath , or air hunger, is the subjective symptom of breathlessness.It is a normal symptom of heavy exertion but becomes pathological if it occurs in unexpected situations...
and paralysis
Paralysis
Paralysis is loss of muscle function for one or more muscles. Paralysis can be accompanied by a loss of feeling in the affected area if there is sensory damage as well as motor. A study conducted by the Christopher & Dana Reeve Foundation, suggests that about 1 in 50 people have been diagnosed...
. This may be due to its ability to block potassium ion channels which are critical to the proper function of the nervous system. However, individual responses to barium salts vary widely, with some being able to handle barium nitrate casually without problems, and others becoming ill from working with it in small quantities. For example, barium acetate
Barium acetate
Barium acetate is the salt of barium and acetic acid.-Preparation:Barium acetate is generally produced by the reaction of acetic acid with barium carbonate:The reaction is performed in solution and the barium acetate crystallizes out...
was used by Marie Robards to poison her father in 1993.
Non-toxicity of barium sulfate
Because it is highly insoluble in water as well as stomach acids, barium sulfate can be taken oralMouth
The mouth is the first portion of the alimentary canal that receives food andsaliva. The oral mucosa is the mucous membrane epithelium lining the inside of the mouth....
ly. It is eliminated completely from the digestive tract. Unlike other heavy metals
Heavy metals
A heavy metal is a member of a loosely-defined subset of elements that exhibit metallic properties. It mainly includes the transition metals, some metalloids, lanthanides, and actinides. Many different definitions have been proposed—some based on density, some on atomic number or atomic weight,...
, barium does not bioaccumulate
Bioaccumulation
Bioaccumulation refers to the accumulation of substances, such as pesticides, or other organic chemicals in an organism. Bioaccumulation occurs when an organism absorbs a toxic substance at a rate greater than that at which the substance is lost...
. However, inhaled dust containing barium compounds can accumulate in the lungs, causing a benign
Benign
A benign tumor is a tumor that lacks the ability to metastasize. Common examples of benign tumors include moles and uterine fibroids.The term "benign" implies a mild and nonprogressive disease. Indeed, many kinds of benign tumors are harmless to human health...
condition called baritosis
Baritosis
Baritosis is a benign type of pneumoconiosis, which is caused by long-term exposure to barium dust.Barium has a high radio-opacity and the disease may develop after few months of exposure. Extremely dense, discrete small opacities of 2–4 mm diameter, sometimes of a star-like configuration, are...
.

