
Xenon difluoride
Encyclopedia
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent
inorganic fluoride
s it is moisture sensitive. It decomposes
on contact with light
or water vapour. Xenon difluoride is a dense, white crystal
line solid. It has a nauseating odour and low vapor pressure
. It has a strong characteristic infrared
doublet at 550 cm−1
and 556 cm−1.
molecule with an Xe–F bond length of 197.73±0.15 pm
in the vapor stage, and 200 pm in the solid phase. The packing arrangement in solid shows that the fluorine atoms of neighbouring molecules avoid the equatorial region of each molecule. This agrees with the prediction of VSEPR theory, which predicts that there are 3 pairs of non-bonding electrons around the equatorial region of the xenon atom.
At high pressures, novel, non-molecular forms of xenon difluoride can be obtained. Under a pressure of ~50 GPa, transforms into a semiconductor consisting of units linked in a two-dimensional structure, like graphite
. At even higher pressures, above 70 GPa, it becomes metallic, forming a three-dimensional structure containing units.
The reaction requires heat, irradiation, or an electrical discharge. The product is gas
eous, but can be condensed
at −30 °C. It is purified by fractional distillation
or selective condensation using a vacuum line.
The first published report of XeF2 was in October 1962 by Chernick, et al. However, though published later, XeF2 was probably first created by Rudolf Hoppe
at the University of Münster
, Germany, in early 1962, by reacting fluorine and xenon gas mixtures in an electrical discharge. Shortly after these reports, Weeks, Cherwick, and Matheson of Argonne National Laboratory
reported the synthesis of XeF2 using an all-nickel system with transparent alumina windows, in which equal parts Xe and F2 gases react at low pressure upon irradiation by an ultraviolet
source to give XeF2. Williamson reported that the reaction works equally well at atmospheric pressure in a dry Pyrex
glass bulb using sunlight as a source. It was noted that the synthesis worked even on cloudy days.
In the previous syntheses the F2 reactant had been purified to remove HF. Šmalc and Lutar found that if this step is skipped the reaction rate actually proceeds at four times the original rate.
In 1965, it was also synthesized by reacting xenon gas with dioxygen difluoride
.
,
, anhydrous HF
and
, without reduction or oxidation. Solubility in HF is high, at 167g per 100g HF at 29.95°C.
The usual precautions associated with use of F2 are required: grease-free, preferably fluorine passivated metal system or very dry glassware. Air must be excluded to preclude formation of xenon trioxide
, an explosive (this is only true if the XeF2 sample contains XeF4 which hydrolyzes to xenon trioxide).
to generate radicals
and passing the gas over . The resulting waxy white solid decomposes completely within 4 hours at room temperature.
The XeF+ cation is formed by combining xenon difluoride with a strong fluoride acceptor, such as an excess of liquid antimony pentafluoride
:
Adding xenon gas to this pale yellow solution at a pressure of 2-3 atm
produces a green solution containing the paramagnetic ion, which contains a Xe−Xe bond: ("apf" denotes solution in liquid )
This reaction is reversible; removing xenon gas from the solution causes the ion to revert back to xenon gas and , and the color of the solution returns to a pale yellow.
In the presence of liquid HF
, dark green crystals can be precipitated from the green solution at −30°C:
X-ray crystallography
indicates that the Xe-Xe bond length in this compound is 309 pm
, indicating a very weak bond. The ion is isoelectronic
with the ion, which is also dark green.
in coordination complexes
of transition metals. For example, in HF solution:
Crystallographic analysis shows that the magnesium atom is coordinated to 6 fluorine atoms. Four of the fluorines are attributed to the four xenon difluoride ligands while the other two are a pair of cis-AsF ligands.
A similar reaction is:
In the crystal structure of this product the magnesium atom is octahedrally-coordinated
and the XeF2 ligands are axial while the AsF ligands are equatorial.
Many such reactions with products of the form [Mx(XeF2)n](AF6)x have been observed, where M can be Ca, Sr, Ba, Pb, Ag, La, or Nd and A can be As, Sb or P.
Recently, a compound was synthesised where a metal atom was coordinated solely by XeF2 fluorine atoms:
This reaction requires a large excess of xenon difluoride. The structure of the salt is such that half of the Ca2+ ions are coordinated by fluorine atoms from xenon difluoride, while the other Ca2+ ions are coordinated by both XeF2 and AsF.
Among the fluorination reactions that xenon difluoride undergoes are:
is selective about which atom it fluorinates, making it a useful reagent for fluorinating heteroatoms without touching other substituents in organic compounds. For example, it fluorinates the arsenic atom in trimethylarsine, but leaves the methyl group
s untouched:
will also oxidatively decarboxylate carboxylic acid
s to the corresponding fluoroalkanes
:
Silicon tetrafluoride
has been found to act as a catalyst in fluorination by .
for silicon
, particularly in the production of microelectromechanical systems
, (MEMS). Brazzle, Dokmeci, et al., describe this process:
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
inorganic fluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
s it is moisture sensitive. It decomposes
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
on contact with light
Photodissociation
Photodissociation, photolysis, or photodecomposition is a chemical reaction in which a chemical compound is broken down by photons. It is defined as the interaction of one or more photons with one target molecule....
or water vapour. Xenon difluoride is a dense, white crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
line solid. It has a nauseating odour and low vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
. It has a strong characteristic infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
doublet at 550 cm−1
Wavenumber
In the physical sciences, the wavenumber is a property of a wave, its spatial frequency, that is proportional to the reciprocal of the wavelength. It is also the magnitude of the wave vector...
and 556 cm−1.
Structure
Xenon difluoride is a linearLinear molecular geometry
In chemistry, the Linear molecular geometry describes the arrangement of three or more atoms placed at an expected bond angle of 180º. Linear organic molecules, e.g. acetylene, are often described by invoking sp orbital hybridization for the carbon centers. Many linear molecules exist, prominent...
molecule with an Xe–F bond length of 197.73±0.15 pm
Picometre
A picometre is a unit of length in the metric system, equal to one trillionth, i.e. of a metre, which is the current SI base unit of length...
in the vapor stage, and 200 pm in the solid phase. The packing arrangement in solid shows that the fluorine atoms of neighbouring molecules avoid the equatorial region of each molecule. This agrees with the prediction of VSEPR theory, which predicts that there are 3 pairs of non-bonding electrons around the equatorial region of the xenon atom.
At high pressures, novel, non-molecular forms of xenon difluoride can be obtained. Under a pressure of ~50 GPa, transforms into a semiconductor consisting of units linked in a two-dimensional structure, like graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
. At even higher pressures, above 70 GPa, it becomes metallic, forming a three-dimensional structure containing units.
Synthesis
Synthesis proceeds by the simple formula:- Xe + F2 → XeF2
The reaction requires heat, irradiation, or an electrical discharge. The product is gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous, but can be condensed
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
at −30 °C. It is purified by fractional distillation
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
or selective condensation using a vacuum line.
The first published report of XeF2 was in October 1962 by Chernick, et al. However, though published later, XeF2 was probably first created by Rudolf Hoppe
Rudolf Hoppe
Rudolf Hoppe , a German chemist, discovered the first covalent noble gas compounds.-Academic career:...
at the University of Münster
University of Münster
The University of Münster is a public university located in the city of Münster, North Rhine-Westphalia in Germany. The WWU is part of the Deutsche Forschungsgemeinschaft, a society of Germany's leading research universities...
, Germany, in early 1962, by reacting fluorine and xenon gas mixtures in an electrical discharge. Shortly after these reports, Weeks, Cherwick, and Matheson of Argonne National Laboratory
Argonne National Laboratory
Argonne National Laboratory is the first science and engineering research national laboratory in the United States, receiving this designation on July 1, 1946. It is the largest national laboratory by size and scope in the Midwest...
reported the synthesis of XeF2 using an all-nickel system with transparent alumina windows, in which equal parts Xe and F2 gases react at low pressure upon irradiation by an ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
source to give XeF2. Williamson reported that the reaction works equally well at atmospheric pressure in a dry Pyrex
Pyrex
Pyrex is a brand name for glassware, introduced by Corning Incorporated in 1915.Originally, Pyrex was made from borosilicate glass. In the 1940s the composition was changed for some products to tempered soda-lime glass, which is the most common form of glass used in glass bakeware in the US and has...
glass bulb using sunlight as a source. It was noted that the synthesis worked even on cloudy days.
In the previous syntheses the F2 reactant had been purified to remove HF. Šmalc and Lutar found that if this step is skipped the reaction rate actually proceeds at four times the original rate.
In 1965, it was also synthesized by reacting xenon gas with dioxygen difluoride
Dioxygen difluoride
Dioxygen difluoride is a compound with the formula . It exists as an orange solid that melts into a red liquid at −163 °C It is a strong oxidant and decomposes into and oxygen even at −160 °C .-Preparation:...
.
Solubility
is soluble in solvents such as ,Bromine trifluoride
Bromine trifluoride is an interhalogen compound with the formula BrF3. This toxic, colourless, and corrosive liquid is soluble in sulfuric acid but explodes on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent...
,
Iodine pentafluoride
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is a fluoride of iodine. It is a colourless or yellow liquid with a density of 3.250 g cm−3. It was first synthesized by Henri Moissan in 1891 by burning solid iodine in fluorine gas...
, anhydrous HF
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
and
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
, without reduction or oxidation. Solubility in HF is high, at 167g per 100g HF at 29.95°C.
Safety considerations
Xenon difluoride (XeF2) is most easily made directly from xenon and fluorine. An evacuated glass container of fluorine and xenon is exposed to daylight.The usual precautions associated with use of F2 are required: grease-free, preferably fluorine passivated metal system or very dry glassware. Air must be excluded to preclude formation of xenon trioxide
Xenon trioxide
Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly , accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials...
, an explosive (this is only true if the XeF2 sample contains XeF4 which hydrolyzes to xenon trioxide).
Derived xenon compounds
Other xenon compounds may be derived from xenon difluoride. The unstable organoxenon compound can be made by irradiating hexafluoroethaneHexafluoroethane
Hexafluoroethane is a fluorocarbon counterpart to the hydrocarbon ethane. It is a non-flammable gas negligibly soluble in water and slightly soluble in alcohol.-Physical properties:...
to generate radicals
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
and passing the gas over . The resulting waxy white solid decomposes completely within 4 hours at room temperature.
The XeF+ cation is formed by combining xenon difluoride with a strong fluoride acceptor, such as an excess of liquid antimony pentafluoride
Antimony pentafluoride
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a valuable Lewis acid and a component of the superacid fluoroantimonic acid, the strongest known acid...
:
- + → +
Adding xenon gas to this pale yellow solution at a pressure of 2-3 atm
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
produces a green solution containing the paramagnetic ion, which contains a Xe−Xe bond: ("apf" denotes solution in liquid )
- 3 Xe (g) + (apf) + (l) 2 (apf) + (apf)
This reaction is reversible; removing xenon gas from the solution causes the ion to revert back to xenon gas and , and the color of the solution returns to a pale yellow.
In the presence of liquid HF
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
, dark green crystals can be precipitated from the green solution at −30°C:
- (apf) + 4 (apf) → (s) + 3 (apf)
X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
indicates that the Xe-Xe bond length in this compound is 309 pm
Picometre
A picometre is a unit of length in the metric system, equal to one trillionth, i.e. of a metre, which is the current SI base unit of length...
, indicating a very weak bond. The ion is isoelectronic
Isoelectronicity
Two or more molecular entities are described as being isoelectronic with each other if they have the same number of electrons or a similar electron configuration and the same structure , regardless of the nature of the elements involved.The term valence isoelectronic is used when these molecular...
with the ion, which is also dark green.
Coordination chemistry
XeF2 can act as a ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in coordination complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
of transition metals. For example, in HF solution:
- Mg(AsF6)2 + 4 XeF2 → [Mg(XeF2)4](AsF6)2
Crystallographic analysis shows that the magnesium atom is coordinated to 6 fluorine atoms. Four of the fluorines are attributed to the four xenon difluoride ligands while the other two are a pair of cis-AsF ligands.
A similar reaction is:
- Mg(AsF6)2 + 2 XeF2 → [Mg(XeF2)2](AsF6)2
In the crystal structure of this product the magnesium atom is octahedrally-coordinated
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
and the XeF2 ligands are axial while the AsF ligands are equatorial.
Many such reactions with products of the form [Mx(XeF2)n](AF6)x have been observed, where M can be Ca, Sr, Ba, Pb, Ag, La, or Nd and A can be As, Sb or P.
Recently, a compound was synthesised where a metal atom was coordinated solely by XeF2 fluorine atoms:
- 2 Ca(AsF6 )2 + 9 XeF2 → Ca2(XeF2)9(AsF6)4.
This reaction requires a large excess of xenon difluoride. The structure of the salt is such that half of the Ca2+ ions are coordinated by fluorine atoms from xenon difluoride, while the other Ca2+ ions are coordinated by both XeF2 and AsF.
As a fluorinating agent
Xenon difluoride is a strong fluorinating and oxidising agent. With fluoride ion acceptors, it forms XeF and species which are even more powerful fluorinators.Among the fluorination reactions that xenon difluoride undergoes are:
- Oxidative fluorination:
-
- Ph3TeF + XeF2 → Ph3TeF3 + Xe
- Reductive fluorination:
- 2 CrO2F2 + XeF2 → 2 CrOF3 + Xe +O2
- Aromatic fluorination:
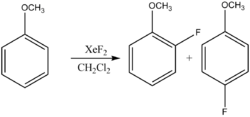
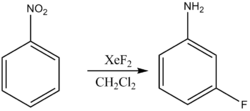
- Alkene fluorination:
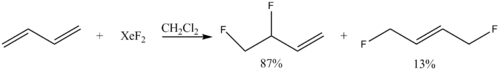
- Ph3TeF + XeF2 → Ph3TeF3 + Xe
is selective about which atom it fluorinates, making it a useful reagent for fluorinating heteroatoms without touching other substituents in organic compounds. For example, it fluorinates the arsenic atom in trimethylarsine, but leaves the methyl group
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
s untouched:
- + →
will also oxidatively decarboxylate carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s to the corresponding fluoroalkanes
Haloalkane
The haloalkanes are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and...
:
- RCOOH + XeF2 → RF + CO2 + Xe + HF
Silicon tetrafluoride
Silicon tetrafluoride
Silicon tetrafluoride or Tetrafluorosilane is the chemical compound with the formula SiF4. This tetrahedral molecule is notable for having a remarkably narrow liquid range...
has been found to act as a catalyst in fluorination by .
As an etchant
Xenon difluoride is also used as an isotropic gaseous etchantEtching
Etching is the process of using strong acid or mordant to cut into the unprotected parts of a metal surface to create a design in intaglio in the metal...
for silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, particularly in the production of microelectromechanical systems
Microelectromechanical systems
Microelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
, (MEMS). Brazzle, Dokmeci, et al., describe this process:
The mechanism of the etch is as follows. First, the XeF2 adsorbs and dissociates to xenon (Xe) and fluorine (F) on the surface of silicon. Fluorine is the main etchant in the silicon etching process. The reaction describing the silicon with XeF2 is
- 2 XeF2 + Si → 2 Xe + SiF4
XeF2 has a relatively high etch rate and does not require ion bombardment or external energy sources in order to etch silicon.

