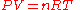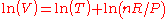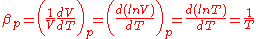Thermal expansion
Encyclopedia
Thermal expansion is the tendency of matter to change in volume
in response to a change in temperature
.
When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is limited in size, and only occurs within limited temperature ranges (see examples below). The degree of expansion divided by the change in temperature is called the material's coefficient of thermal expansion and generally varies with temperature.
is available, it can be used to predict the values of the thermal expansion at all the required temperatures and pressure
s, along with many other state function
s.
, rather than "thermal contraction". For example, the coefficient of thermal expansion of water drops to zero as it is cooled to roughly 4 °C and then becomes negative below this temperature; this means that water has a maximum density
at this temperature, and this leads to bodies of water maintaining this temperature at their lower depths during extended periods of sub-zero weather. Also, fairly pure silicon has a negative coefficient of thermal expansion for temperatures between about 18 kelvin
and 120 kelvin.
Thermal expansion generally decreases with increasing bond energy, which also has an effect on the hardness of solids, so, harder materials are more likely to have lower thermal expansion. In general, liquids expand slightly more than solids. The thermal expansion of glasses is higher compared to that of crystals. At the glass transition temperature, rearrangements that occur in an amorphous material lead to characteristic discontinuities of coefficient of thermal expansion or specific heat. These discontinuities allow detection of the glass transition temperature where a supercooled liquid transforms to a glass.
Absorption
or desorption of water (or other solvents) can change the size of many common materials; many organic materials change size much more due to this effect than they do to thermal expansion. Common plastics exposed to water can, in the long term, expand many percent.
The volumetric thermal expansion coefficient is the most basic thermal expansion coefficient. In general, substances expand or contract when their temperature changes, with expansion or contraction occurring in all directions. Substances that expand at the same rate in every direction are called isotropic
. For isotropic materials, the area and linear coefficients may be calculated from the volumetric coefficient.
Mathematical definitions of these coefficients are defined below for solids, liquids, and gasses.
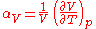
The subscript p indicates that the pressure is held constant during the expansion, and the subscript "V" stresses that it is the volumetric (not linear) expansion that enters this general definition. In the case of a gas, the fact that the pressure is held constant is important, because the volume of a gas will vary appreciably with pressure as well as temperature. For a gas of low density this can be seen from the ideal gas
law.
materials, the pressure does not appreciably affect the size of an object, and so, for solids, it's usually not necessary to specify that the pressure be held constant.
Common engineering solids usually have coefficients of thermal expansion that do not vary significantly over the range of temperatures where they are designed to be used, so where extremely high accuracy is not required, practical calculations can be based on a constant, average, value of the coefficient of expansion.
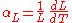
where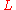 is the linear dimension (e.g. length) and
is the linear dimension (e.g. length) and 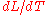 is the rate of change of that linear dimension per unit change in temperature.
is the rate of change of that linear dimension per unit change in temperature.
The change in the linear dimension can be estimated to be:
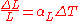
This equation works well as long as the linear expansion coefficient does not change much over the change in temperature . If it does, the equation must be integrated.
. If it does, the equation must be integrated.
, given by and defined as:
and defined as:
where is the length before the change of temperature and
is the length before the change of temperature and 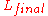 is the length after the change of temperature.
is the length after the change of temperature.
For most solids, thermal expansion is proportional to the change in temperature:
Thus, the change in either the strain
or temperature can be estimated by:
where
is the difference of the temperature between the two recorded strains, measured in degrees Celsius
or kelvin
s,
and
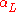 is the linear coefficient of thermal expansion in inverse kelvins.
is the linear coefficient of thermal expansion in inverse kelvins.

where is some area of interest on the object, and
is some area of interest on the object, and 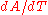 is the rate of change of that area per unit change in temperature.
is the rate of change of that area per unit change in temperature.
The change in the linear dimension can be estimated as: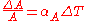
This equation works well as long as the linear expansion coefficient does not change much over the change in temperature . If it does, the equation must be integrated.
. If it does, the equation must be integrated.

where is the volume of the material, and
is the volume of the material, and 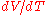 is the rate of change of that volume with temperature.
is the rate of change of that volume with temperature.
This means that the volume of a material changes by some fixed fractional amount. For example, a steel block with a volume of 1 cubic meter might expand to 1.002 cubic meters when the temperature is raised by 50 °C. This is an expansion of 0.2%. If we had a block of steel with a volume of 2 cubic meters, then under the same conditions, it would expand to 2.004 cubic meters, again an expansion of 0.2%. The volumetric expansion coefficient would be 0.2% for 50 °C, or 0.004% per degree C.
If we already know the expansion coefficient, then we can calculate the change in volume
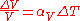
where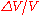 is the fractional change in volume (e.g., 0.002) and
is the fractional change in volume (e.g., 0.002) and  is the change in temperature (50 C).
is the change in temperature (50 C).
The above example assumes that the expansion coefficient did not change as the temperature changed. This is not always true, but for small changes in temperature, it is a good approximation. If the volumetric expansion coefficient does change appreciably with temperature, then the above equation will have to be integrated:
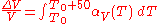
where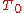 is the starting temperature and
is the starting temperature and 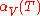 is the volumetric expansion coefficient as a function of temperature T.
is the volumetric expansion coefficient as a function of temperature T.

This ratio arises because volume is composed of three mutually orthogonal directions. Thus, in an isotropic material, for small differential changes, one-third of the volumetric expansion is in a single axis. As an example, take a cube of steel that has sides of length L. The original volume will be and the new volume, after a temperature increase, will be
and the new volume, after a temperature increase, will be

We can make the substitutions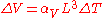 and, for isotropic materials,
and, for isotropic materials, 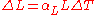 . We now have:
. We now have:
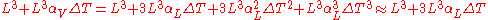
Since the volumetric and linear coefficients are defined only for extremely small temperature and dimensional changes (that is, when and
and  are small), the last two terms can be ignored and we get the above relationship between the two coefficients. If we are trying to go back and forth between volumetric and linear coefficients using larger values of
are small), the last two terms can be ignored and we get the above relationship between the two coefficients. If we are trying to go back and forth between volumetric and linear coefficients using larger values of  then we will need to take into account the third term, and sometimes even the fourth term.
then we will need to take into account the third term, and sometimes even the fourth term.
Similarly, the area thermal expansion coefficient is 2/3 of the volumetric coefficient.

This ratio can be found in a way similar to that in the linear example above, noting that the area of a face on the cube is just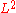 . Also, the same considerations must be made when dealing with large values of
. Also, the same considerations must be made when dealing with large values of  .
.
, will generally have different linear expansion coefficients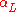 in different directions. As a result, the total volumetric expansion is distributed unequally among the three axes. If the crystal symmetry is monoclinic or triclinic, even the angles between these axes are subject to thermal changes. In such cases it is necessary to treat the coefficient of thermal expansion as a tensor
in different directions. As a result, the total volumetric expansion is distributed unequally among the three axes. If the crystal symmetry is monoclinic or triclinic, even the angles between these axes are subject to thermal changes. In such cases it is necessary to treat the coefficient of thermal expansion as a tensor
with up to six independent elements. A good way to determine the elements of the tensor is to study the expansion by powder diffraction.
, the volumetric thermal expansivity (i.e. relative change in volume due to temperature change) depends on the type of process in which temperature is changed. Two known cases are isobaric
change, where pressure
is held constant, and adiabatic change, where no work is done and no change in entropy
occurs.
In an isobaric
process, the volumetric thermal expansivity, which we denote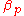 , is:
, is:
The index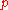 denotes an isobaric process.
denotes an isobaric process.
Thermal expansion is also used in mechanical applications to fit parts over one another, e.g. a bushing can be fitted over a shaft by making its inner diameter slightly smaller than the diameter of the shaft, then heating it until it fits over the shaft, and allowing it to cool after it has been pushed over the shaft, thus achieving a 'shrink fit'. Induction shrink fitting
is a common industrial method to pre-heat metal components between 150 °C and 300 °C thereby causing them to expand and allow for the insertion or removal of another component.
There exist some alloys with a very small linear expansion coefficient, used in applications that demand very small changes in physical dimension over a range of temperatures. One of these is Invar
36, with α approximately equal to 0.6×10−6/°C. These alloys are useful in aerospace applications where wide temperature swings may occur.
Pullinger's apparatus is used to determine the linear expansion of a metallic rod in the laboratory. The apparatus consists of a metal cylinder closed at both ends (called a steam jacket). It is provided with an inlet and outlet for the steam. The steam for heating the rod is supplied by a boiler which is connected by a rubber tube to the inlet. The center of the cylinder contains a hole to insert a thermometer. The rod under investigation is enclosed in a steam jacket. One of its ends is free, but the other end is pressed against a fixed screw. The position of the rod is determined by a micrometer screw gauge or spherometer
.
The control of thermal expansion in ceramics is a key concern for a wide range of reasons. For example, ceramics are brittle and cannot tolerate sudden changes in temperature (without cracking) if their expansion is too high. Ceramics need to be joined or work in consort with a wide range of materials and therefore their expansion must be matched to the application. Because glazes need to be firmly attached to the underlying porcelain (or other body type) their thermal expansion must be tuned to 'fit' the body so that crazing
or shivering do not occur. Good example of products whose thermal expansion is the key to their success are CorningWare
and the spark plug
. The thermal expansion of ceramic bodies can be controlled by firing to create crystalline species that will influence the overall expansion of the material in the desired direction. In addition or instead the formulation of the body can employ materials delivering particles of the desired expansion to the matrix. The thermal expansion of glazes is controlled by their ceramic chemistry
. In most cases there are complex issues involved in controlling body and glaze expansion, adjusting for thermal expansion must be done with an eye to other properties that will be affected, generally trade-offs are required.
Heat-induced expansion has to be taken into account in most areas of engineering. A few examples are:
Thermometer
s are another application of thermal expansion — most contain a liquid (usually mercury or alcohol) which is constrained to flow in only one direction (along the tube) due to changes in volume brought about by changes in temperature. A bi-metal mechanical thermometer uses a bimetallic strip and bends due to the differing thermal expansion of the two metals.
In the table below, the range for α is from 10−7/°C for hard solids to 10−3/°C for organic liquids. α varies with the temperature and some materials have a very high variation.
Theoretically, the coefficient of linear expansion can be approximated from the coefficient of volumetric expansion (β≈3α). However, for liquids, α is calculated through the experimental determination of β, so it is more accurate to state β here, rather than α.
(The formula β≈3α is usually used for solids.)
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
in response to a change in temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
.
When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is limited in size, and only occurs within limited temperature ranges (see examples below). The degree of expansion divided by the change in temperature is called the material's coefficient of thermal expansion and generally varies with temperature.
Predicting expansion
If an equation of stateEquation of state
In physics and thermodynamics, an equation of state is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions...
is available, it can be used to predict the values of the thermal expansion at all the required temperatures and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
s, along with many other state function
State function
In thermodynamics, a state function, function of state, state quantity, or state variable is a property of a system that depends only on the current state of the system, not on the way in which the system acquired that state . A state function describes the equilibrium state of a system...
s.
Contraction effects
A number of materials contract on heating within certain temperature ranges; this is usually called negative thermal expansionNegative thermal expansion
Negative Thermal Expansion is a physicochemical process in which some materials contract upon heating rather than expanding as most materials do. Materials which undergo this unusual process have a range of potential engineering, photonic, electronic, and structural applications...
, rather than "thermal contraction". For example, the coefficient of thermal expansion of water drops to zero as it is cooled to roughly 4 °C and then becomes negative below this temperature; this means that water has a maximum density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
at this temperature, and this leads to bodies of water maintaining this temperature at their lower depths during extended periods of sub-zero weather. Also, fairly pure silicon has a negative coefficient of thermal expansion for temperatures between about 18 kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
and 120 kelvin.
Factors affecting thermal expansion
Unlike gases or liquids, solid materials tend to keep their shape when undergoing thermal expansion.Thermal expansion generally decreases with increasing bond energy, which also has an effect on the hardness of solids, so, harder materials are more likely to have lower thermal expansion. In general, liquids expand slightly more than solids. The thermal expansion of glasses is higher compared to that of crystals. At the glass transition temperature, rearrangements that occur in an amorphous material lead to characteristic discontinuities of coefficient of thermal expansion or specific heat. These discontinuities allow detection of the glass transition temperature where a supercooled liquid transforms to a glass.
Absorption
Sorption
Sorption refers to the action of absorption* Absorption is the incorporation of a substance in one state into another of a different state ....
or desorption of water (or other solvents) can change the size of many common materials; many organic materials change size much more due to this effect than they do to thermal expansion. Common plastics exposed to water can, in the long term, expand many percent.
Coefficient of thermal expansion
The coefficient of thermal expansion describes how the size of an object changes with a change in temperature. Specifically, it measures the fractional change in size per degree change in temperature at a constant pressure. Several types of coefficients have been developed: volumetric, area, and linear. Which is used depends on the particular application and which dimensions are considered important. For solids, one might only be concerned with the change along a length, or over some area.The volumetric thermal expansion coefficient is the most basic thermal expansion coefficient. In general, substances expand or contract when their temperature changes, with expansion or contraction occurring in all directions. Substances that expand at the same rate in every direction are called isotropic
Isotropy
Isotropy is uniformity in all orientations; it is derived from the Greek iso and tropos . Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix an, hence anisotropy. Anisotropy is also used to describe situations where properties vary...
. For isotropic materials, the area and linear coefficients may be calculated from the volumetric coefficient.
Mathematical definitions of these coefficients are defined below for solids, liquids, and gasses.
General volumetric thermal expansion coefficient
In the general case of a gas, liquid, or solid, the volumetric coefficient of thermal expansion is given by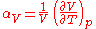
The subscript p indicates that the pressure is held constant during the expansion, and the subscript "V" stresses that it is the volumetric (not linear) expansion that enters this general definition. In the case of a gas, the fact that the pressure is held constant is important, because the volume of a gas will vary appreciably with pressure as well as temperature. For a gas of low density this can be seen from the ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
law.
Expansion in solids
Materials generally change their size when subjected to a temperature change while the pressure is held constant. In the special case of solidSolid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
materials, the pressure does not appreciably affect the size of an object, and so, for solids, it's usually not necessary to specify that the pressure be held constant.
Common engineering solids usually have coefficients of thermal expansion that do not vary significantly over the range of temperatures where they are designed to be used, so where extremely high accuracy is not required, practical calculations can be based on a constant, average, value of the coefficient of expansion.
Linear expansion
The linear thermal expansion coefficient relates the change in a material's linear dimensions to a change in temperature. It is the fractional change in length per degree of temperature change. Ignoring pressure, we may write: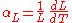
where
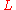 is the linear dimension (e.g. length) and
is the linear dimension (e.g. length) and 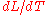 is the rate of change of that linear dimension per unit change in temperature.
is the rate of change of that linear dimension per unit change in temperature.The change in the linear dimension can be estimated to be:
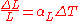
This equation works well as long as the linear expansion coefficient does not change much over the change in temperature
 . If it does, the equation must be integrated.
. If it does, the equation must be integrated.Effects on strain
For solid materials with a significant length, like rods or cables, an estimate of the amount of thermal expansion can be described by the material strainStrain (materials science)
In continuum mechanics, the infinitesimal strain theory, sometimes called small deformation theory, small displacement theory, or small displacement-gradient theory, deals with infinitesimal deformations of a continuum body...
, given by
 and defined as:
and defined as:
where
 is the length before the change of temperature and
is the length before the change of temperature and 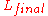 is the length after the change of temperature.
is the length after the change of temperature.For most solids, thermal expansion is proportional to the change in temperature:

Thus, the change in either the strain
Strain (materials science)
In continuum mechanics, the infinitesimal strain theory, sometimes called small deformation theory, small displacement theory, or small displacement-gradient theory, deals with infinitesimal deformations of a continuum body...
or temperature can be estimated by:

where

is the difference of the temperature between the two recorded strains, measured in degrees Celsius
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
or kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
s,
and
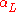 is the linear coefficient of thermal expansion in inverse kelvins.
is the linear coefficient of thermal expansion in inverse kelvins.Area expansion
The area thermal expansion coefficient relates the change in a material's area dimensions to a change in temperature. It is the fractional change in area per degree of temperature change. Ignoring pressure, we may write:
where
 is some area of interest on the object, and
is some area of interest on the object, and 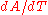 is the rate of change of that area per unit change in temperature.
is the rate of change of that area per unit change in temperature.The change in the linear dimension can be estimated as:
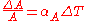
This equation works well as long as the linear expansion coefficient does not change much over the change in temperature
 . If it does, the equation must be integrated.
. If it does, the equation must be integrated.Volumetric expansion
For a solid, we can ignore the effects of pressure on the material, and the volumetric thermal expansion coefficient can be written :
where
 is the volume of the material, and
is the volume of the material, and 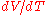 is the rate of change of that volume with temperature.
is the rate of change of that volume with temperature.This means that the volume of a material changes by some fixed fractional amount. For example, a steel block with a volume of 1 cubic meter might expand to 1.002 cubic meters when the temperature is raised by 50 °C. This is an expansion of 0.2%. If we had a block of steel with a volume of 2 cubic meters, then under the same conditions, it would expand to 2.004 cubic meters, again an expansion of 0.2%. The volumetric expansion coefficient would be 0.2% for 50 °C, or 0.004% per degree C.
If we already know the expansion coefficient, then we can calculate the change in volume
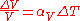
where
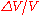 is the fractional change in volume (e.g., 0.002) and
is the fractional change in volume (e.g., 0.002) and  is the change in temperature (50 C).
is the change in temperature (50 C).The above example assumes that the expansion coefficient did not change as the temperature changed. This is not always true, but for small changes in temperature, it is a good approximation. If the volumetric expansion coefficient does change appreciably with temperature, then the above equation will have to be integrated:
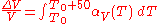
where
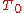 is the starting temperature and
is the starting temperature and 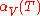 is the volumetric expansion coefficient as a function of temperature T.
is the volumetric expansion coefficient as a function of temperature T.Isotropic materials
For exactly isotropic materials, and for small expansions, the linear thermal expansion coefficient is one third the volumetric coefficient.
This ratio arises because volume is composed of three mutually orthogonal directions. Thus, in an isotropic material, for small differential changes, one-third of the volumetric expansion is in a single axis. As an example, take a cube of steel that has sides of length L. The original volume will be
 and the new volume, after a temperature increase, will be
and the new volume, after a temperature increase, will be
We can make the substitutions
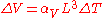 and, for isotropic materials,
and, for isotropic materials, 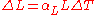 . We now have:
. We now have: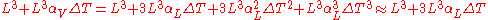
Since the volumetric and linear coefficients are defined only for extremely small temperature and dimensional changes (that is, when
 and
and  are small), the last two terms can be ignored and we get the above relationship between the two coefficients. If we are trying to go back and forth between volumetric and linear coefficients using larger values of
are small), the last two terms can be ignored and we get the above relationship between the two coefficients. If we are trying to go back and forth between volumetric and linear coefficients using larger values of  then we will need to take into account the third term, and sometimes even the fourth term.
then we will need to take into account the third term, and sometimes even the fourth term.Similarly, the area thermal expansion coefficient is 2/3 of the volumetric coefficient.

This ratio can be found in a way similar to that in the linear example above, noting that the area of a face on the cube is just
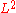 . Also, the same considerations must be made when dealing with large values of
. Also, the same considerations must be made when dealing with large values of  .
.Anisotropic materials
Materials with anisotropic structures, such as crystals and many compositesComposite material
Composite materials, often shortened to composites or called composition materials, are engineered or naturally occurring materials made from two or more constituent materials with significantly different physical or chemical properties which remain separate and distinct at the macroscopic or...
, will generally have different linear expansion coefficients
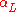 in different directions. As a result, the total volumetric expansion is distributed unequally among the three axes. If the crystal symmetry is monoclinic or triclinic, even the angles between these axes are subject to thermal changes. In such cases it is necessary to treat the coefficient of thermal expansion as a tensor
in different directions. As a result, the total volumetric expansion is distributed unequally among the three axes. If the crystal symmetry is monoclinic or triclinic, even the angles between these axes are subject to thermal changes. In such cases it is necessary to treat the coefficient of thermal expansion as a tensorTensor
Tensors are geometric objects that describe linear relations between vectors, scalars, and other tensors. Elementary examples include the dot product, the cross product, and linear maps. Vectors and scalars themselves are also tensors. A tensor can be represented as a multi-dimensional array of...
with up to six independent elements. A good way to determine the elements of the tensor is to study the expansion by powder diffraction.
Expansion in gases
For an ideal gasIdeal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
, the volumetric thermal expansivity (i.e. relative change in volume due to temperature change) depends on the type of process in which temperature is changed. Two known cases are isobaric
Isobaric
Isobaric may refer to:*in thermodynamics, an isobaric process, i.e. one that is carried out at constant pressure;...
change, where pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
is held constant, and adiabatic change, where no work is done and no change in entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
occurs.
In an isobaric
Isobaric
Isobaric may refer to:*in thermodynamics, an isobaric process, i.e. one that is carried out at constant pressure;...
process, the volumetric thermal expansivity, which we denote
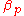 , is:
, is:
The index
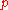 denotes an isobaric process.
denotes an isobaric process.Expansion in liquids
Theoretically, the coefficient of linear expansion can be found from the coefficient of volumetric expansion (β≈3α). However, for liquids, α is calculated through the experimental determination of β.Apparent and Absolute Expansion
When measuring the expansion of a liquid, the measurement must account for the expansion of the container as well. For example, a flask, that has been constructed with a long narrow stem filled with enough liquid that the stem itself is partially filled, when placed in a heat bath will initially show the column of liquid in the stem to drop followed by the immediate increase of that column until the flask/liquid/heat bath system has thermalized. The initial observation of the column of liquid dropping is not due to an initial contraction of the liquid but rather the expansion of the flask as it contacts the heat bath first. Soon after, the liquid in the flask is heated by the flask itself and begins to expand. Since liquids typically have a greater expansion over solids the liquid in the flask eventually exceeds that of the flask causing the column of liquid in the flask to rise. A direct measurement of the height of the liquid column is a measurement of the Apparent Expansion of the liquid. The Absolute expansion of the liquid is the apparent expansion corrected for the expansion of the containing vessel.Examples and Applications
The expansion and contraction of materials must be considered when designing large structures, when using tape or chain to measure distances for land surveys, when designing molds for casting hot material, and in other engineering applications when large changes in dimension due to temperature are expected.Thermal expansion is also used in mechanical applications to fit parts over one another, e.g. a bushing can be fitted over a shaft by making its inner diameter slightly smaller than the diameter of the shaft, then heating it until it fits over the shaft, and allowing it to cool after it has been pushed over the shaft, thus achieving a 'shrink fit'. Induction shrink fitting
Induction shrink fitting
Induction shrink fitting refers to the use of induction heater technology to pre-heat metal components between and thereby causing them to expand and allow for the insertion or removal of another component. Typically the lower temperature range is used on metals such as aluminium and higher...
is a common industrial method to pre-heat metal components between 150 °C and 300 °C thereby causing them to expand and allow for the insertion or removal of another component.
There exist some alloys with a very small linear expansion coefficient, used in applications that demand very small changes in physical dimension over a range of temperatures. One of these is Invar
Invar
Invar, also known generically as FeNi36 , is a nickel steel alloy notable for its uniquely low coefficient of thermal expansion . The name, Invar, comes from the word invariable, referring to its lack of expansion or contraction with temperature changes.It was invented in 1896 by Swiss scientist...
36, with α approximately equal to 0.6×10−6/°C. These alloys are useful in aerospace applications where wide temperature swings may occur.
Pullinger's apparatus is used to determine the linear expansion of a metallic rod in the laboratory. The apparatus consists of a metal cylinder closed at both ends (called a steam jacket). It is provided with an inlet and outlet for the steam. The steam for heating the rod is supplied by a boiler which is connected by a rubber tube to the inlet. The center of the cylinder contains a hole to insert a thermometer. The rod under investigation is enclosed in a steam jacket. One of its ends is free, but the other end is pressed against a fixed screw. The position of the rod is determined by a micrometer screw gauge or spherometer
Spherometer
A spherometer is an instrument for the precise measurement of the radius of a sphere. Originally, these instruments were primarily used by opticians to measure the curvature of the surface of a lens....
.
The control of thermal expansion in ceramics is a key concern for a wide range of reasons. For example, ceramics are brittle and cannot tolerate sudden changes in temperature (without cracking) if their expansion is too high. Ceramics need to be joined or work in consort with a wide range of materials and therefore their expansion must be matched to the application. Because glazes need to be firmly attached to the underlying porcelain (or other body type) their thermal expansion must be tuned to 'fit' the body so that crazing
Crazing
Crazing is a network of fine cracks on the surface of a material, for example in a glaze layer.Crazing is a phenomenon that frequently precedes fracture in some glassy thermoplastic polymers. Crazing occurs in regions of high hydrostatic tension, or in regions of very localized yielding, which...
or shivering do not occur. Good example of products whose thermal expansion is the key to their success are CorningWare
Corningware
CorningWare was originally a brand name for a unique pyroceramic glass cookware resistant to thermal shock, that was first introduced in 1958 by Corning Glass Works. CorningWare is notable for the fact that it can be used directly on the stovetop.- History :In 1953, Dr. S...
and the spark plug
Spark plug
A spark plug is an electrical device that fits into the cylinder head of some internal combustion engines and ignites compressed fuels such as aerosol, gasoline, ethanol, and liquefied petroleum gas by means of an electric spark.Spark plugs have an insulated central electrode which is connected by...
. The thermal expansion of ceramic bodies can be controlled by firing to create crystalline species that will influence the overall expansion of the material in the desired direction. In addition or instead the formulation of the body can employ materials delivering particles of the desired expansion to the matrix. The thermal expansion of glazes is controlled by their ceramic chemistry
Ceramic chemistry
Ceramic chemistry is a branch of inorganic chemistry that studies the relationship between the physical properties of fired ceramic glazes and their chemistry...
. In most cases there are complex issues involved in controlling body and glaze expansion, adjusting for thermal expansion must be done with an eye to other properties that will be affected, generally trade-offs are required.
Heat-induced expansion has to be taken into account in most areas of engineering. A few examples are:
- Metal framed windows need rubber spacers
- Rubber tires
- Metal hot water heating pipes should not be used in long straight lengths
- Large structures such as railways and bridges need expansion jointExpansion jointAn expansion joint or movement joint is an assembly designed to safely absorb the heat-induced expansion and contraction of various construction materials, to absorb vibration, to hold certain parts together, or to allow movement due to ground settlement or earthquakes...
s in the structures to avoid sun kinkSun kinkSun kink refers to a condition that can occur on hot days in rail tracks. The phenomenon is caused by what is properly termed as buckling.The buckling force in the track due to warming up is a function of the rise in temperature only and is independent of the track length: F = E A \alpha_L \Delta... - One of the reasons for the poor performance of cold car engines is that parts have inefficiently large spacings until the normal operating temperatureOperating temperatureAn operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
is achieved. - A gridiron pendulumGridiron pendulumThe gridiron pendulum was an improved clock pendulum invented by British clockmaker John Harrison around 1726. It didn't change its effective length with temperature, so its period of swing stayed constant with changes in ambient temperature...
uses an arrangement of different metals to maintain a more temperature stable pendulum length. - A power line on a hot day is droopy, but on a cold day it is tight. This is because the metals expand under heat.
Thermometer
Thermometer
Developed during the 16th and 17th centuries, a thermometer is a device that measures temperature or temperature gradient using a variety of different principles. A thermometer has two important elements: the temperature sensor Developed during the 16th and 17th centuries, a thermometer (from the...
s are another application of thermal expansion — most contain a liquid (usually mercury or alcohol) which is constrained to flow in only one direction (along the tube) due to changes in volume brought about by changes in temperature. A bi-metal mechanical thermometer uses a bimetallic strip and bends due to the differing thermal expansion of the two metals.
Thermal expansion coefficients for various materials
This section summarizes the coefficients for some common materials.In the table below, the range for α is from 10−7/°C for hard solids to 10−3/°C for organic liquids. α varies with the temperature and some materials have a very high variation.
Theoretically, the coefficient of linear expansion can be approximated from the coefficient of volumetric expansion (β≈3α). However, for liquids, α is calculated through the experimental determination of β, so it is more accurate to state β here, rather than α.
(The formula β≈3α is usually used for solids.)
| Material | Linear coefficient, α, at 20 °C (10−6/°C) |
Volumetric coefficient, β, at 20 °C (10−6/°C) |
Notes |
|---|---|---|---|
| Aluminium Aluminium Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances.... |
23 | 69 | |
| Benzocyclobutene Benzocyclobutene Benzocyclobutene is a benzene ring fused to a cyclobutane ring. It has chemical formula 88.BCB is frequently used to create photosensitive polymers. BCB-based polymer dielectrics may be spun on or applied to various substrates for use in Micro Electro-Mechanical Systems and microelectronics... |
42 | 126 | |
| Brass Brass Brass is an alloy of copper and zinc; the proportions of zinc and copper can be varied to create a range of brasses with varying properties.In comparison, bronze is principally an alloy of copper and tin... |
19 | 57 | |
| Carbon steel | 10.8 | 32.4 | |
| Concrete Concrete Concrete is a composite construction material, composed of cement and other cementitious materials such as fly ash and slag cement, aggregate , water and chemical admixtures.The word concrete comes from the Latin word... |
12 | 36 | |
| Copper Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish... |
17 | 51 | |
| Diamond Diamond In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions... |
1 | 3 | |
| Ethanol Ethanol Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a... |
250 | 750 | Linear value is approximate |
| Gallium(III) arsenide Gallium(III) arsenide Gallium arsenide is a compound of the elements gallium and arsenic. It is a III/V semiconductor, and is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated circuits, infrared light-emitting diodes, laser diodes, solar cells and... |
5.8 | 17.4 | |
| Gasoline Gasoline Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain... |
317 | 950 | Linear value is approximate |
| Glass Glass Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives... |
8.5 | 25.5 | |
| Glass Glass Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives... , borosilicate |
3.3 | 9.9 | |
| Gold Gold Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a... |
14 | 42 | |
| Indium phosphide | 4.6 | 13.8 | |
| Invar Invar Invar, also known generically as FeNi36 , is a nickel steel alloy notable for its uniquely low coefficient of thermal expansion . The name, Invar, comes from the word invariable, referring to its lack of expansion or contraction with temperature changes.It was invented in 1896 by Swiss scientist... |
1.2 | 3.6 | |
| Iron | 11.8 | 33.3 | |
| Kapton Kapton Kapton is a polyimide film developed by DuPont which can remain stable in a wide range of temperatures, from -273 to +400 °C... |
20 | 60 | DuPont™ Kapton® 200EN |
| Lead Lead Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed... |
29 | 87 | |
| MACOR MACOR MACOR is a machineable glass-ceramic developed and sold by Corning Incorporated. It is a white material that looks somewhat like porcelain. MACOR has excellent thermal characteristics, acting as efficient insulation, and stable up to temperatures of 1000 °C, with very little thermal expansion or... |
9.3 | ||
| Magnesium Magnesium Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole... |
26 | 78 | |
| Mercury Mercury (element) Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum... |
61 | 182 | Linear value is approximate |
| Molybdenum Molybdenum Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores... |
4.8 | 14.4 | |
| Nickel Nickel Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile... |
13 | 39 | |
| Oak Oak An oak is a tree or shrub in the genus Quercus , of which about 600 species exist. "Oak" may also appear in the names of species in related genera, notably Lithocarpus... |
54 | 162 | Perpendicular to the grain |
| Pine Pine Pines are trees in the genus Pinus ,in the family Pinaceae. They make up the monotypic subfamily Pinoideae. There are about 115 species of pine, although different authorities accept between 105 and 125 species.-Etymology:... |
34 | 102 | Perpendicular to the grain |
| Platinum Platinum Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal... |
9 | 27 | |
| PVC PVC Polyvinyl chloride is a plastic.PVC may also refer to:*Param Vir Chakra, India's highest military honor*Peripheral venous catheter, a small, flexible tube placed into a peripheral vein in order to administer medication or fluids... |
52 | 156 | |
| Quartz Quartz Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,... (fused Fused quartz Fused quartz and fused silica are types of glass containing primarily silica in amorphous form. They are manufactured using several different processes... ) |
0.59 | 1.77 | |
| Rubber Rubber Natural rubber, also called India rubber or caoutchouc, is an elastomer that was originally derived from latex, a milky colloid produced by some plants. The plants would be ‘tapped’, that is, an incision made into the bark of the tree and the sticky, milk colored latex sap collected and refined... |
77 | 231 | |
| Sapphire Sapphire Sapphire is a gemstone variety of the mineral corundum, an aluminium oxide , when it is a color other than red or dark pink; in which case the gem would instead be called a ruby, considered to be a different gemstone. Trace amounts of other elements such as iron, titanium, or chromium can give... |
5.3 | Parallel to C axis, or [001] | |
| Silicon Carbide Silicon carbide Silicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive... |
2.77 | 8.31 | |
| Silicon Silicon Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table... |
3 | 9 | |
| Silver Silver Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal... |
18 | 54 | |
| Sitall Sitall Sitall aka Sitall CO-115M or Astrositall, is a crystalline glass ceramic material with ultra low coefficient of thermal expansion . It was originally manufactured in the former Soviet Union and was used in the making of primary mirrors for the Russian Maksutov telescopes, but since dissolution has... |
0.15 | 0.45 | |
| Stainless steel Stainless steel In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass.... |
17.3 | 51.9 | |
| Steel Steel Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten... |
11.0 ~ 13.0 | 33.0 ~ 39.0 | Depends on composition |
| Titanium Titanium Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color.... |
8.6 | ||
| Tungsten Tungsten Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as... |
4.5 | 13.5 | |
| Water Water Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a... |
69 | 207 | Linear value is approximate |
| YbGaGe YbGaGe YbGaGe is an alloy of Ytterbium, Gallium and Germanium. It is a zero thermal expansion material but is conductive. It can maintain nearly the same volume between 100 and 400 K. Such materials have application in space and other environments where low thermal expansion materials are required.... |
≐0 | ≐0 |
External links
- Glass Thermal Expansion Thermal expansion measurement, definitions, thermal expansion calculation from the glass composition
- Water Expansion Calculator Water thermal expansion calculator
- DoITPoMS Teaching and Learning Package on Thermal Expansion and the Bi-material Strip
- Engineering Toolbox – List of coefficients of Linear Expansion for some common materials
- Article on how β is determined
- MatWeb: Free database of engineering properties for over 79,000 materials
- Clemson University Physics Lab: Linear Thermal Expansion
- USA NIST Website - Temperature and Dimensional Measurement workshop
- Hyperphysics: Thermal expansion
- Understanding Thermal Expansion in Ceramic Glazes