
Old quantum theory
Encyclopedia
The old quantum theory was a collection of results from the years 1900–1925 which predate modern quantum mechanics
. The theory was never complete or self-consistent, but was a collection of heuristic
prescriptions which are now understood to be the first quantum corrections to classical mechanics
. The Bohr model
was the focus of study, and Arnold Sommerfeld
made a crucial contribution by quantizing the z-component of the angular momentum, which in the old quantum era was inappropriately called space quantization (Richtungsquantelung). This allowed the orbits of the electron to be ellipses instead of circles, and introduced the concept of quantum degeneracy. The theory would have correctly explained the Zeeman effect
, except for the issue of electron spin
.
The main tool was Bohr Sommerfeld quantization, a procedure for selecting out certain discrete set of states of a classical integrable motion as allowed states. These are like the allowed orbits of the Bohr model of the atom; the system can only be in one of these states and not in any states in between. The theory did not extend to chaotic motions, because it required a full multiply periodic trajectory of the classical system for all time in order to pose the quantum conditions.
except that not every motion is allowed, only those motions which obey the old quantum condition:
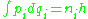
where the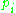 are the momenta of the system and the
are the momenta of the system and the 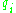 are the corresponding coordinates. The quantum numbers
are the corresponding coordinates. The quantum numbers 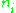 are integers and the integral is taken over one period of the motion. The integral is an area in phase space, which is a quantity called the action, which is quantized in units of Planck's constant. For this reason, Planck's constant was often called the quantum of action.
are integers and the integral is taken over one period of the motion. The integral is an area in phase space, which is a quantity called the action, which is quantized in units of Planck's constant. For this reason, Planck's constant was often called the quantum of action.
In order for the old quantum condition to make sense, the classical motion must be separable, meaning that there are separate coordinates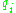 in terms of which the motion is periodic. The periods of the different motions do not have to be the same, they can even be incommensurate, but there must be a set of coordinates where the motion decomposes in a multi-periodic way.
in terms of which the motion is periodic. The periods of the different motions do not have to be the same, they can even be incommensurate, but there must be a set of coordinates where the motion decomposes in a multi-periodic way.
The motivation for the old quantum condition was the correspondence principle
, complemented by the physical observation that the quantities which are quantized must be adiabatic invariant
s. Given Planck's quantization rule for the harmonic oscillator, either condition determines the correct classical quantity to quantize in a general system up to an additive constant.
, whose Hamiltonian
is:
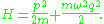
The level sets of H are the orbits, and the quantum condition is that the area enclosed by an orbit in phase space is an integer. It follows that the energy is quantized according to the Planck rule:
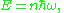
a result which was known well before, and used to formulate the old quantum condition.
The thermal properties of a quantized oscillator may be found by averaging the energy in each of the discrete states assuming that they are occupied with a Boltzmann weight:
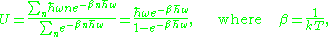
kT is Boltzmann constant times the absolute temperature
, which is the temperature as measured in more natural units of energy. The quantity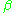 is more fundamental in thermodynamics than the temperature, because it is the thermodynamic potential associated to the energy.
is more fundamental in thermodynamics than the temperature, because it is the thermodynamic potential associated to the energy.
From this expression, it is easy to see that for large values of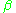 , for very low temperatures, the average energy U in the Harmonic oscillator approaches zero very quickly, exponentially fast. The reason is that kT is the typical energy of random motion at temperature T, and when this is smaller than
, for very low temperatures, the average energy U in the Harmonic oscillator approaches zero very quickly, exponentially fast. The reason is that kT is the typical energy of random motion at temperature T, and when this is smaller than 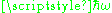 , there is not enough energy to give the oscillator even one quantum of energy. So the oscillator stays in its ground state, storing next to no energy at all.
, there is not enough energy to give the oscillator even one quantum of energy. So the oscillator stays in its ground state, storing next to no energy at all.
This means that at very cold temperatures, the change in energy with respect to beta, or equivalently the change in energy with respect to temperature, is also exponentially small. The change in energy with respect to temperature is the specific heat, so the specific heat is exponentially small at low temperatures, going to zero like
At small values of , at high temperatures, the average energy U is equal to
, at high temperatures, the average energy U is equal to 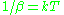 . This reproduces the equipartition theorem
. This reproduces the equipartition theorem
of classical thermodynamics--- every harmonic oscillator at temperature T has energy kT on average. This means that the specific heat of an oscillator is constant in classical mechanics and equal to k. For a collection of atoms connected by springs, a reasonable model of a solid, the total specific heat is equal to the total number of oscillators times k. There are overall three oscillators for each atom, corresponding to the three possible directions of independent oscillations in three dimensions. So the specific heat of a classical solid is always 3k per atom, or in chemistry units, 3R per mole
of atoms.
Monatomic solids at room temperatures have approximately the same specific heat of 3k per atom, but at low temperatures they don't. The specific heat is smaller at colder temperatures, and it goes to zero at absolute zero. This is true for all material systems, and this observation is called the third law of thermodynamics
. Classical mechanics cannot explain the third law, because in classical mechanics the specific heat is independent of the temperature.
This contradiction between classical mechanics and the specific heat of cold materials was noted by James Clerk Maxwell
in the 19th century, and remained a deep puzzle for those who advocated an atomic theory of matter. Einstein resolved this problem in 1906 by proposing that atomic motion is quantized. This was the first application of quantum theory to mechanical systems. A short while later, Debye gave a quantitative theory of solid specific heats
in terms of quantized oscillators with various frequencies (see Einstein solid
and Debye model
).
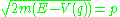
which is integrated over all values of q between the classical turning points, the places where the momentum vanishes. The integral is easiest for a particle in a box of length L, where the quantum condition is:
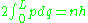
which gives the allowed momenta:
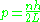
and the energy levels
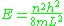
Another easy case to solve with the old quantum theory is a linear potential on the positive halfline, the constant confining force F binding a particle to an impenetrable wall. This case is much more difficult in the full quantum mechanical treatment, and unlike the other examples, the semiclassical answer here is not exact but approximate, becoming more accurate at large quantum numbers.

so that the quantum condition is:
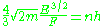
Which determines the energy levels.
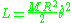
which determines that the momentum J conjugate to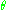 , the polar angle,
, the polar angle, 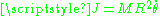 . The old quantum condition requires that J multiplied by the period of
. The old quantum condition requires that J multiplied by the period of 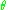 is an integer multiple of Planck's constant:
is an integer multiple of Planck's constant:
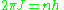
the angular momentum to be an integer multiple of . In the Bohr model
. In the Bohr model
, this restriction imposed on circular orbits was enough to determine the energy levels.
In three dimensions, a rigid rotator can be described by two angles — and
and  , where
, where  is the inclination relative to an arbitrarily chosen z-axis while
is the inclination relative to an arbitrarily chosen z-axis while 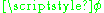 is the rotator angle in the projection to the x–y plane. The kinetic energy is again the only contribution to the Lagrangian:
is the rotator angle in the projection to the x–y plane. The kinetic energy is again the only contribution to the Lagrangian:
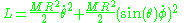
And the conjugate momenta are and
and 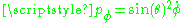 . The equation of motion for
. The equation of motion for 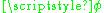 is trivial:
is trivial:  is a constant:
is a constant:
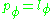
which is the z-component of the angular momentum. The quantum condition demands that the integral of the constant as
as 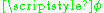 varies from 0 to
varies from 0 to 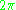 is an integer multiple of h:
is an integer multiple of h:
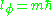
And m is called the magnetic quantum number
, because the z component of the angular momentum is the magnetic moment of the rotator along the z direction in the case where the particle at the end of the rotator is charged.
Since the three dimensional rotator is rotating about an axis, the total angular momentum should be restricted in the same way as the two-dimensional rotator. The two quantum conditions restrict the total angular momentum and the z-component of the angular momentum to be the integers l,m. This condition is reproduced in modern quantum mechanics, but in the era of the old quantum theory it led to a paradox: how can the orientation of the angular momentum relative to the arbitrarily chosen z-axis be quantized? This seems to pick out a direction in space.
This phenomenon, the quantization of angular momentum about an axis, was given the name space quantization, because it seemed incompatible with rotational invariance. In modern quantum mechanics, the angular momentum is quantized the same way, but the discrete states of definite angular momentum in any one orientation are quantum superposition
s of the states in other orientations, so that the process of quantization does not pick out a preferred axis. For this reason, the name "space quantization" fell out of favor, and the same phenomenon is now called the quantization of angular momentum.
For a fixed value of the total angular momentum L, the Hamiltonian for a classical Kepler problem is (the unit of mass and unit of energy redefined to absorb two constants):

Fixing the energy to be constant and solving for the radial momentum p, the quantum condition integral is:
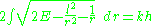
which is elementary, and gives a new quantum number k which determines the energy in combination with l. The energy is:
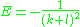
and it only depends on the sum of k and l, which is the principal quantum number n. Since k is positive, the allowed values of l for any given n are no bigger than n. The energies reproduce those in the Bohr model
, except with the correct quantum mechanical multiplicities, with some ambiguity at the extreme values.
The semiclassical hydrogen atom is called the Sommerfeld
model, and its orbits are ellipses of various sizes at discrete inclinations. The Sommerfeld model predicted that the magnetic moment of an atom measured along an axis will only take on discrete values, a result which seems to contradict rotational invariance but which was confirmed by the Stern–Gerlach experiment
.
Bohr–Sommerfeld theory is a part of the development of quantum mechanics
and describes the possibility of atomic energy levels being split by a magnetic field
.
derived the relativistic solution of atomic energy levels. We will start this derivation with the relativistic equation for energy in the electric potential
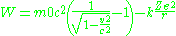
After substitution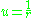 we get
we get
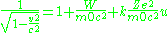
For momentum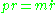 ,
, 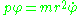 and their ratio
and their ratio 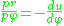 the equation of motion is (see Binet equation
the equation of motion is (see Binet equation
)
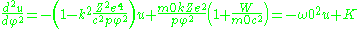
with solution
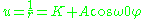
The angular shift of periapsis per revolution is given by
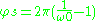
With the quantum conditions
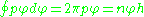
and
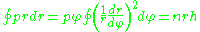
we will obtain energies
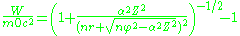
where is the fine-structure constant
is the fine-structure constant
. This solution is same as the solution of the Dirac equation
.
(see page 139/140).
, particles of light, and named them photon
s
Einstein's theoretical argument was based on thermodynamics
, on counting the number of states, and so was not completely convincing. Nevertheless, he concluded that light had attributes of both waves and particles, more precisely that an electromagnetic standing wave with frequency with the quantized energy:
with the quantized energy:
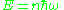
should be thought of as consisting of n photons each with an energy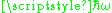 . Einstein could not describe how the photons were related to the wave.
. Einstein could not describe how the photons were related to the wave.
The photons have momentum as well as energy, and the momentum had to be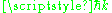 where
where 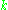 is the wavenumber of the electromagnetic wave. This is required by relativity, because the momentum and energy form a four-vector
is the wavenumber of the electromagnetic wave. This is required by relativity, because the momentum and energy form a four-vector
, as do the frequency and wave-number.
In 1924, as a PhD candidate, Louis de Broglie proposed a new interpretation of the quantum condition. He suggested that all matter, electrons as well as photons, are described by waves obeying the relations.

or, expressed in terms of wavelength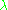 instead,
instead,
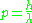
He then noted that the quantum condition:
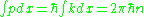
counts the change in phase for the wave as it travels along the classical orbit, and requires that it be an integer multiple of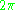 . Expressed in wavelengths, the number of wavelengths along a classical orbit must be an integer. This is the condition for constructive interference, and it explained the reason for quantized orbits—the matter waves make standing waves only at discrete frequencies, at discrete energies.
. Expressed in wavelengths, the number of wavelengths along a classical orbit must be an integer. This is the condition for constructive interference, and it explained the reason for quantized orbits—the matter waves make standing waves only at discrete frequencies, at discrete energies.
For example, for a particle confined in a box, a standing wave must fit an integer number of wavelengths between twice the distance between the walls. The condition becomes:
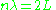
so that the quantized momenta are:
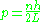
reproducing the old quantum energy levels.
This development was given a more mathematical form by Einstein, who noted that the phase function for the waves: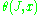 in a mechanical system should be identified with the solution to the Hamilton–Jacobi equation
in a mechanical system should be identified with the solution to the Hamilton–Jacobi equation
, an equation which even Hamilton
considered to be a short-wavelength limit of a wave mechanics.
These ideas led to the development of Schrödinger equation
.
Kramers suggested that the orbits of a quantum system should be Fourier analyzed, decomposed into harmonics at multiples of the orbit frequency:
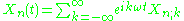
The index n describes the quantum numbers of the orbit, it would be n–l–m in the Sommerfeld model. The frequency is the angular frequency of the orbit
is the angular frequency of the orbit 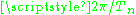 while k is an index for the Fourier mode. Bohr had suggested that the k-th harmonic of the classical motion correspond to the transition from level n to level n−k.
while k is an index for the Fourier mode. Bohr had suggested that the k-th harmonic of the classical motion correspond to the transition from level n to level n−k.
Kramers proposed that the transition between states were analogous to classical emission of radiation, which happens at frequencies at multiples of the orbit frequencies. The rate of emission of radiation is proportional to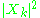 , as it would be in classical mechanics. The description was approximate, since the Fourier components did not have frequencies that exactly match the energy spacings between levels.
, as it would be in classical mechanics. The description was approximate, since the Fourier components did not have frequencies that exactly match the energy spacings between levels.
This idea led to the development of matrix mechanics
.
on the emission and absorption of light, and began in earnest after the work of Albert Einstein
on the specific heats of solids. Einstein, followed by Debye, applied quantum principles to the motion of atoms, explaining the specific heat anomaly.
In 1913, Niels Bohr
identified the correspondence principle
and used it to formulate a model
of the Hydrogen atom
which explained the line spectrum. In the next few years Arnold Sommerfeld
extended the quantum rule to arbitrary integrable systems making use of the principle of adiabatic invariance
of the quantum numbers introduced by Lorentz and Einstein. Sommerfeld's model was much closer to the modern quantum mechanical picture than Bohr's.
Throughout the 1910s and well into the 1920s, many problems were attacked using the old quantum theory with mixed results. Molecular rotation and vibration spectra were understood and the electron's spin was discovered, leading to the confusion of half-integer quantum numbers. Max Planck
introduced the zero point energy and Arnold Sommerfeld
semiclassically quantized the relativistic hydrogen atom. Hendrik Kramers explained the Stark effect
. Bose
and Einstein gave the correct quantum statistics for photons.
Kramers gave a prescription for calculating transition probabilities between quantum states in terms of Fourier components of the motion, ideas which were extended in collaboration with Werner Heisenberg
to a semiclassical matrix-like description of atomic transition probabilities. Heisenberg went on to reformulate all of quantum theory in terms of a version of these transition matrices, creating Matrix mechanics
.
In 1924, Louis de Broglie introduced the wave theory of matter, which was extended to a semiclassical equation for matter waves by Albert Einstein a short time later. In 1926 Erwin Schrödinger
found a completely quantum mechanical wave-equation, which reproduced all the successes of the old quantum theory without ambiguities and inconsistencies. Schrödinger's wave mechanics developed separately from matrix mechanics until Schrödinger and others proved that the two methods predicted the same experimental consequences. Paul Dirac later proved in 1926 that both methods can be obtained from a more general method called transformation theory
.
Matrix mechanics and wave mechanics put an end to the era of the old-quantum theory.
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
. The theory was never complete or self-consistent, but was a collection of heuristic
Heuristic
Heuristic refers to experience-based techniques for problem solving, learning, and discovery. Heuristic methods are used to speed up the process of finding a satisfactory solution, where an exhaustive search is impractical...
prescriptions which are now understood to be the first quantum corrections to classical mechanics
Classical mechanics
In physics, classical mechanics is one of the two major sub-fields of mechanics, which is concerned with the set of physical laws describing the motion of bodies under the action of a system of forces...
. The Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
was the focus of study, and Arnold Sommerfeld
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and groomed a large number of students for the new era of theoretical physics...
made a crucial contribution by quantizing the z-component of the angular momentum, which in the old quantum era was inappropriately called space quantization (Richtungsquantelung). This allowed the orbits of the electron to be ellipses instead of circles, and introduced the concept of quantum degeneracy. The theory would have correctly explained the Zeeman effect
Zeeman effect
The Zeeman effect is the splitting of a spectral line into several components in the presence of a static magnetic field. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field...
, except for the issue of electron spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
.
The main tool was Bohr Sommerfeld quantization, a procedure for selecting out certain discrete set of states of a classical integrable motion as allowed states. These are like the allowed orbits of the Bohr model of the atom; the system can only be in one of these states and not in any states in between. The theory did not extend to chaotic motions, because it required a full multiply periodic trajectory of the classical system for all time in order to pose the quantum conditions.
Basic principles
The basic idea of the old quantum theory is that the motion in an atomic system is quantized, or discrete. The system obeys classical mechanicsClassical mechanics
In physics, classical mechanics is one of the two major sub-fields of mechanics, which is concerned with the set of physical laws describing the motion of bodies under the action of a system of forces...
except that not every motion is allowed, only those motions which obey the old quantum condition:
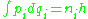
where the
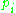 are the momenta of the system and the
are the momenta of the system and the 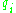 are the corresponding coordinates. The quantum numbers
are the corresponding coordinates. The quantum numbers 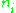 are integers and the integral is taken over one period of the motion. The integral is an area in phase space, which is a quantity called the action, which is quantized in units of Planck's constant. For this reason, Planck's constant was often called the quantum of action.
are integers and the integral is taken over one period of the motion. The integral is an area in phase space, which is a quantity called the action, which is quantized in units of Planck's constant. For this reason, Planck's constant was often called the quantum of action.In order for the old quantum condition to make sense, the classical motion must be separable, meaning that there are separate coordinates
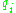 in terms of which the motion is periodic. The periods of the different motions do not have to be the same, they can even be incommensurate, but there must be a set of coordinates where the motion decomposes in a multi-periodic way.
in terms of which the motion is periodic. The periods of the different motions do not have to be the same, they can even be incommensurate, but there must be a set of coordinates where the motion decomposes in a multi-periodic way.The motivation for the old quantum condition was the correspondence principle
Correspondence principle
In physics, the correspondence principle states that the behavior of systems described by the theory of quantum mechanics reproduces classical physics in the limit of large quantum numbers....
, complemented by the physical observation that the quantities which are quantized must be adiabatic invariant
Adiabatic invariant
An adiabatic invariant is a property of a physical system that stays constant when changes occur slowly.In thermodynamics, an adiabatic process is a change that occurs without heat flow, and slowly compared to the time to reach equilibrium. In an adiabatic process, the system is in equilibrium at...
s. Given Planck's quantization rule for the harmonic oscillator, either condition determines the correct classical quantity to quantize in a general system up to an additive constant.
Harmonic oscillator
The simplest system in the old quantum theory is the harmonic oscillatorHarmonic oscillator
In classical mechanics, a harmonic oscillator is a system that, when displaced from its equilibrium position, experiences a restoring force, F, proportional to the displacement, x: \vec F = -k \vec x \, where k is a positive constant....
, whose Hamiltonian
Hamiltonian (quantum mechanics)
In quantum mechanics, the Hamiltonian H, also Ȟ or Ĥ, is the operator corresponding to the total energy of the system. Its spectrum is the set of possible outcomes when one measures the total energy of a system...
is:
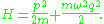
The level sets of H are the orbits, and the quantum condition is that the area enclosed by an orbit in phase space is an integer. It follows that the energy is quantized according to the Planck rule:
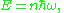
a result which was known well before, and used to formulate the old quantum condition.
The thermal properties of a quantized oscillator may be found by averaging the energy in each of the discrete states assuming that they are occupied with a Boltzmann weight:
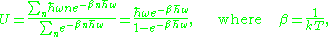
kT is Boltzmann constant times the absolute temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
, which is the temperature as measured in more natural units of energy. The quantity
 is more fundamental in thermodynamics than the temperature, because it is the thermodynamic potential associated to the energy.
is more fundamental in thermodynamics than the temperature, because it is the thermodynamic potential associated to the energy.From this expression, it is easy to see that for large values of
 , for very low temperatures, the average energy U in the Harmonic oscillator approaches zero very quickly, exponentially fast. The reason is that kT is the typical energy of random motion at temperature T, and when this is smaller than
, for very low temperatures, the average energy U in the Harmonic oscillator approaches zero very quickly, exponentially fast. The reason is that kT is the typical energy of random motion at temperature T, and when this is smaller than 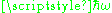 , there is not enough energy to give the oscillator even one quantum of energy. So the oscillator stays in its ground state, storing next to no energy at all.
, there is not enough energy to give the oscillator even one quantum of energy. So the oscillator stays in its ground state, storing next to no energy at all.This means that at very cold temperatures, the change in energy with respect to beta, or equivalently the change in energy with respect to temperature, is also exponentially small. The change in energy with respect to temperature is the specific heat, so the specific heat is exponentially small at low temperatures, going to zero like
At small values of
 , at high temperatures, the average energy U is equal to
, at high temperatures, the average energy U is equal to 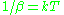 . This reproduces the equipartition theorem
. This reproduces the equipartition theoremEquipartition theorem
In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition...
of classical thermodynamics--- every harmonic oscillator at temperature T has energy kT on average. This means that the specific heat of an oscillator is constant in classical mechanics and equal to k. For a collection of atoms connected by springs, a reasonable model of a solid, the total specific heat is equal to the total number of oscillators times k. There are overall three oscillators for each atom, corresponding to the three possible directions of independent oscillations in three dimensions. So the specific heat of a classical solid is always 3k per atom, or in chemistry units, 3R per mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of atoms.
Monatomic solids at room temperatures have approximately the same specific heat of 3k per atom, but at low temperatures they don't. The specific heat is smaller at colder temperatures, and it goes to zero at absolute zero. This is true for all material systems, and this observation is called the third law of thermodynamics
Third law of thermodynamics
The third law of thermodynamics is a statistical law of nature regarding entropy:For other materials, the residual entropy is not necessarily zero, although it is always zero for a perfect crystal in which there is only one possible ground state.-History:...
. Classical mechanics cannot explain the third law, because in classical mechanics the specific heat is independent of the temperature.
This contradiction between classical mechanics and the specific heat of cold materials was noted by James Clerk Maxwell
James Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
in the 19th century, and remained a deep puzzle for those who advocated an atomic theory of matter. Einstein resolved this problem in 1906 by proposing that atomic motion is quantized. This was the first application of quantum theory to mechanical systems. A short while later, Debye gave a quantitative theory of solid specific heats
in terms of quantized oscillators with various frequencies (see Einstein solid
Einstein solid
The Einstein solid is a model of a solid based on two assumptions:* Each atom in the lattice is an independent 3D quantum harmonic oscillator* All atoms oscillate with the same frequency...
and Debye model
Debye model
In thermodynamics and solid state physics, the Debye model is a method developed by Peter Debye in 1912 for estimating the phonon contribution to the specific heat in a solid. It treats the vibrations of the atomic lattice as phonons in a box, in contrast to the Einstein model, which treats the...
).
One dimensional potential
One dimensional problems are easy to solve. At any energy E, the value of the momentum p is found from the conservation equation: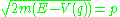
which is integrated over all values of q between the classical turning points, the places where the momentum vanishes. The integral is easiest for a particle in a box of length L, where the quantum condition is:
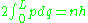
which gives the allowed momenta:
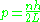
and the energy levels
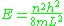
Another easy case to solve with the old quantum theory is a linear potential on the positive halfline, the constant confining force F binding a particle to an impenetrable wall. This case is much more difficult in the full quantum mechanical treatment, and unlike the other examples, the semiclassical answer here is not exact but approximate, becoming more accurate at large quantum numbers.

so that the quantum condition is:
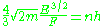
Which determines the energy levels.
Rotator
Another simple system is the rotator. A rotator consists of a mass M at the end of a massless rigid rod of length R and in two dimensions has the Lagrangian: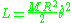
which determines that the momentum J conjugate to
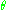 , the polar angle,
, the polar angle, 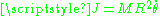 . The old quantum condition requires that J multiplied by the period of
. The old quantum condition requires that J multiplied by the period of 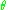 is an integer multiple of Planck's constant:
is an integer multiple of Planck's constant: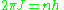
the angular momentum to be an integer multiple of
 . In the Bohr model
. In the Bohr modelBohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
, this restriction imposed on circular orbits was enough to determine the energy levels.
In three dimensions, a rigid rotator can be described by two angles —
 and
and  , where
, where  is the inclination relative to an arbitrarily chosen z-axis while
is the inclination relative to an arbitrarily chosen z-axis while 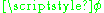 is the rotator angle in the projection to the x–y plane. The kinetic energy is again the only contribution to the Lagrangian:
is the rotator angle in the projection to the x–y plane. The kinetic energy is again the only contribution to the Lagrangian: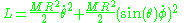
And the conjugate momenta are
 and
and 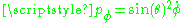 . The equation of motion for
. The equation of motion for 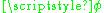 is trivial:
is trivial:  is a constant:
is a constant: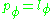
which is the z-component of the angular momentum. The quantum condition demands that the integral of the constant
 as
as 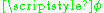 varies from 0 to
varies from 0 to 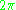 is an integer multiple of h:
is an integer multiple of h: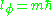
And m is called the magnetic quantum number
Magnetic quantum number
In atomic physics, the magnetic quantum number is the third of a set of quantum numbers which describe the unique quantum state of an electron and is designated by the letter m...
, because the z component of the angular momentum is the magnetic moment of the rotator along the z direction in the case where the particle at the end of the rotator is charged.
Since the three dimensional rotator is rotating about an axis, the total angular momentum should be restricted in the same way as the two-dimensional rotator. The two quantum conditions restrict the total angular momentum and the z-component of the angular momentum to be the integers l,m. This condition is reproduced in modern quantum mechanics, but in the era of the old quantum theory it led to a paradox: how can the orientation of the angular momentum relative to the arbitrarily chosen z-axis be quantized? This seems to pick out a direction in space.
This phenomenon, the quantization of angular momentum about an axis, was given the name space quantization, because it seemed incompatible with rotational invariance. In modern quantum mechanics, the angular momentum is quantized the same way, but the discrete states of definite angular momentum in any one orientation are quantum superposition
Quantum superposition
Quantum superposition is a fundamental principle of quantum mechanics. It holds that a physical system exists in all its particular, theoretically possible states simultaneously; but, when measured, it gives a result corresponding to only one of the possible configurations.Mathematically, it...
s of the states in other orientations, so that the process of quantization does not pick out a preferred axis. For this reason, the name "space quantization" fell out of favor, and the same phenomenon is now called the quantization of angular momentum.
Hydrogen atom
The angular part of the Hydrogen atom is just the rotator, and gives the quantum numbers l and m. The only remaining variable is the radial coordinate, which executes a periodic one dimensional potential motion, which can be solved.For a fixed value of the total angular momentum L, the Hamiltonian for a classical Kepler problem is (the unit of mass and unit of energy redefined to absorb two constants):

Fixing the energy to be constant and solving for the radial momentum p, the quantum condition integral is:
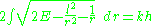
which is elementary, and gives a new quantum number k which determines the energy in combination with l. The energy is:
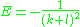
and it only depends on the sum of k and l, which is the principal quantum number n. Since k is positive, the allowed values of l for any given n are no bigger than n. The energies reproduce those in the Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
, except with the correct quantum mechanical multiplicities, with some ambiguity at the extreme values.
The semiclassical hydrogen atom is called the Sommerfeld
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and groomed a large number of students for the new era of theoretical physics...
model, and its orbits are ellipses of various sizes at discrete inclinations. The Sommerfeld model predicted that the magnetic moment of an atom measured along an axis will only take on discrete values, a result which seems to contradict rotational invariance but which was confirmed by the Stern–Gerlach experiment
Stern–Gerlach experiment
Important in the field of quantum mechanics, the Stern–Gerlach experiment, named after Otto Stern and Walther Gerlach, is a 1922 experiment on the deflection of particles, often used to illustrate basic principles of quantum mechanics...
.
Bohr–Sommerfeld theory is a part of the development of quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
and describes the possibility of atomic energy levels being split by a magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
.
Relativistic orbit
Arnold SommerfeldArnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and groomed a large number of students for the new era of theoretical physics...
derived the relativistic solution of atomic energy levels. We will start this derivation with the relativistic equation for energy in the electric potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
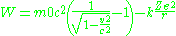
After substitution
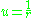 we get
we get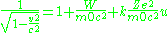
For momentum
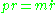 ,
, 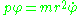 and their ratio
and their ratio 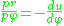 the equation of motion is (see Binet equation
the equation of motion is (see Binet equationBinet equation
The Binet equation, derived by Jacques Philippe Marie Binet, provides the form of a central force given the shape of the orbital motion in plane polar coordinates. The equation can also be used to derive the shape of the orbit for a given force law, but this usually involves the solution to a...
)
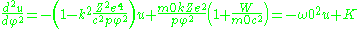
with solution
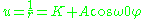
The angular shift of periapsis per revolution is given by
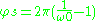
With the quantum conditions
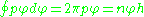
and
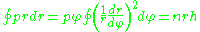
we will obtain energies
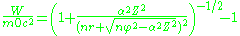
where
 is the fine-structure constant
is the fine-structure constantFine-structure constant
In physics, the fine-structure constant is a fundamental physical constant, namely the coupling constant characterizing the strength of the electromagnetic interaction. Being a dimensionless quantity, it has constant numerical value in all systems of units...
. This solution is same as the solution of the Dirac equation
Dirac equation
The Dirac equation is a relativistic quantum mechanical wave equation formulated by British physicist Paul Dirac in 1928. It provided a description of elementary spin-½ particles, such as electrons, consistent with both the principles of quantum mechanics and the theory of special relativity, and...
.
De Broglie waves
In 1905, Einstein noted that the entropy of the quantized electromagnetic field oscillators in a box is, for short wavelength, equal to the entropy of a gas of point particles in the same box. The number of point particles is equal to the number of quanta. Einstein concluded that the quanta could be treated as if they were localizable objects(see page 139/140).
, particles of light, and named them photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s
Einstein's theoretical argument was based on thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, on counting the number of states, and so was not completely convincing. Nevertheless, he concluded that light had attributes of both waves and particles, more precisely that an electromagnetic standing wave with frequency
 with the quantized energy:
with the quantized energy: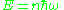
should be thought of as consisting of n photons each with an energy
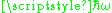 . Einstein could not describe how the photons were related to the wave.
. Einstein could not describe how the photons were related to the wave.The photons have momentum as well as energy, and the momentum had to be
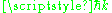 where
where 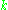 is the wavenumber of the electromagnetic wave. This is required by relativity, because the momentum and energy form a four-vector
is the wavenumber of the electromagnetic wave. This is required by relativity, because the momentum and energy form a four-vectorFour-vector
In the theory of relativity, a four-vector is a vector in a four-dimensional real vector space, called Minkowski space. It differs from a vector in that it can be transformed by Lorentz transformations. The usage of the four-vector name tacitly assumes that its components refer to a standard basis...
, as do the frequency and wave-number.
In 1924, as a PhD candidate, Louis de Broglie proposed a new interpretation of the quantum condition. He suggested that all matter, electrons as well as photons, are described by waves obeying the relations.

or, expressed in terms of wavelength
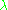 instead,
instead,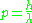
He then noted that the quantum condition:
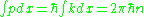
counts the change in phase for the wave as it travels along the classical orbit, and requires that it be an integer multiple of
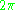 . Expressed in wavelengths, the number of wavelengths along a classical orbit must be an integer. This is the condition for constructive interference, and it explained the reason for quantized orbits—the matter waves make standing waves only at discrete frequencies, at discrete energies.
. Expressed in wavelengths, the number of wavelengths along a classical orbit must be an integer. This is the condition for constructive interference, and it explained the reason for quantized orbits—the matter waves make standing waves only at discrete frequencies, at discrete energies.For example, for a particle confined in a box, a standing wave must fit an integer number of wavelengths between twice the distance between the walls. The condition becomes:
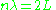
so that the quantized momenta are:
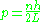
reproducing the old quantum energy levels.
This development was given a more mathematical form by Einstein, who noted that the phase function for the waves:
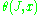 in a mechanical system should be identified with the solution to the Hamilton–Jacobi equation
in a mechanical system should be identified with the solution to the Hamilton–Jacobi equationHamilton–Jacobi equation
In mathematics, the Hamilton–Jacobi equation is a necessary condition describing extremal geometry in generalizations of problems from the calculus of variations. In physics, the Hamilton–Jacobi equation is a reformulation of classical mechanics and, thus, equivalent to other formulations such as...
, an equation which even Hamilton
William Rowan Hamilton
Sir William Rowan Hamilton was an Irish physicist, astronomer, and mathematician, who made important contributions to classical mechanics, optics, and algebra. His studies of mechanical and optical systems led him to discover new mathematical concepts and techniques...
considered to be a short-wavelength limit of a wave mechanics.
These ideas led to the development of Schrödinger equation
Schrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
.
Kramers transition matrix
The old quantum theory was formulated only for special mechanical systems which could be separated into action angle variables which were periodic. It did not deal with the emission and absorption of radiation. Nevertheless, Hendrik Kramers was able to find heuristics for describing how emission and absorption should be calculated.Kramers suggested that the orbits of a quantum system should be Fourier analyzed, decomposed into harmonics at multiples of the orbit frequency:
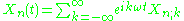
The index n describes the quantum numbers of the orbit, it would be n–l–m in the Sommerfeld model. The frequency
 is the angular frequency of the orbit
is the angular frequency of the orbit 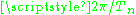 while k is an index for the Fourier mode. Bohr had suggested that the k-th harmonic of the classical motion correspond to the transition from level n to level n−k.
while k is an index for the Fourier mode. Bohr had suggested that the k-th harmonic of the classical motion correspond to the transition from level n to level n−k.Kramers proposed that the transition between states were analogous to classical emission of radiation, which happens at frequencies at multiples of the orbit frequencies. The rate of emission of radiation is proportional to
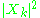 , as it would be in classical mechanics. The description was approximate, since the Fourier components did not have frequencies that exactly match the energy spacings between levels.
, as it would be in classical mechanics. The description was approximate, since the Fourier components did not have frequencies that exactly match the energy spacings between levels.This idea led to the development of matrix mechanics
Matrix mechanics
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925.Matrix mechanics was the first conceptually autonomous and logically consistent formulation of quantum mechanics. It extended the Bohr Model by describing how the quantum jumps...
.
History
The old quantum theory was sparked by the work of Max PlanckMax Planck
Max Karl Ernst Ludwig Planck, ForMemRS, was a German physicist who actualized the quantum physics, initiating a revolution in natural science and philosophy. He is regarded as the founder of the quantum theory, for which he received the Nobel Prize in Physics in 1918.-Life and career:Planck came...
on the emission and absorption of light, and began in earnest after the work of Albert Einstein
Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
on the specific heats of solids. Einstein, followed by Debye, applied quantum principles to the motion of atoms, explaining the specific heat anomaly.
In 1913, Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
identified the correspondence principle
Correspondence principle
In physics, the correspondence principle states that the behavior of systems described by the theory of quantum mechanics reproduces classical physics in the limit of large quantum numbers....
and used it to formulate a model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of the Hydrogen atom
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
which explained the line spectrum. In the next few years Arnold Sommerfeld
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and groomed a large number of students for the new era of theoretical physics...
extended the quantum rule to arbitrary integrable systems making use of the principle of adiabatic invariance
Adiabatic invariant
An adiabatic invariant is a property of a physical system that stays constant when changes occur slowly.In thermodynamics, an adiabatic process is a change that occurs without heat flow, and slowly compared to the time to reach equilibrium. In an adiabatic process, the system is in equilibrium at...
of the quantum numbers introduced by Lorentz and Einstein. Sommerfeld's model was much closer to the modern quantum mechanical picture than Bohr's.
Throughout the 1910s and well into the 1920s, many problems were attacked using the old quantum theory with mixed results. Molecular rotation and vibration spectra were understood and the electron's spin was discovered, leading to the confusion of half-integer quantum numbers. Max Planck
Max Planck
Max Karl Ernst Ludwig Planck, ForMemRS, was a German physicist who actualized the quantum physics, initiating a revolution in natural science and philosophy. He is regarded as the founder of the quantum theory, for which he received the Nobel Prize in Physics in 1918.-Life and career:Planck came...
introduced the zero point energy and Arnold Sommerfeld
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and groomed a large number of students for the new era of theoretical physics...
semiclassically quantized the relativistic hydrogen atom. Hendrik Kramers explained the Stark effect
Stark effect
The Stark effect is the shifting and splitting of spectral lines of atoms and molecules due to presence of an external static electric field. The amount of splitting and or shifting is called the Stark splitting or Stark shift. In general one distinguishes first- and second-order Stark effects...
. Bose
Satyendra Nath Bose
Satyendra Nath Bose FRS was an Indian mathematician and physicist noted for his collaboration with Albert Einstein in developing a theory regarding the gaslike qualities of electromagnetic radiation. He is best known for his work on quantum mechanics in the early 1920s, providing the foundation...
and Einstein gave the correct quantum statistics for photons.
Kramers gave a prescription for calculating transition probabilities between quantum states in terms of Fourier components of the motion, ideas which were extended in collaboration with Werner Heisenberg
Werner Heisenberg
Werner Karl Heisenberg was a German theoretical physicist who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory...
to a semiclassical matrix-like description of atomic transition probabilities. Heisenberg went on to reformulate all of quantum theory in terms of a version of these transition matrices, creating Matrix mechanics
Matrix mechanics
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925.Matrix mechanics was the first conceptually autonomous and logically consistent formulation of quantum mechanics. It extended the Bohr Model by describing how the quantum jumps...
.
In 1924, Louis de Broglie introduced the wave theory of matter, which was extended to a semiclassical equation for matter waves by Albert Einstein a short time later. In 1926 Erwin Schrödinger
Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger was an Austrian physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933...
found a completely quantum mechanical wave-equation, which reproduced all the successes of the old quantum theory without ambiguities and inconsistencies. Schrödinger's wave mechanics developed separately from matrix mechanics until Schrödinger and others proved that the two methods predicted the same experimental consequences. Paul Dirac later proved in 1926 that both methods can be obtained from a more general method called transformation theory
Transformation theory (quantum mechanics)
The term transformation theory refers to a procedure used by P. A. M. Dirac in his early formulation of quantum theory, from around 1927 ....
.
Matrix mechanics and wave mechanics put an end to the era of the old-quantum theory.


