
Lactoylglutathione lyase
Encyclopedia
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
and aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s such as methylglyoxal
Methylglyoxal
Methylglyoxal, also called pyruvaldehyde or 2-oxopropanal is the aldehyde form of pyruvic acid. It has two carbonyl groups, so it is a dicarbonyl compound. Methylglyoxal is both an aldehyde and a ketone....
.
- glutathione + methylglyoxal
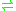 hemithioacetal adduct
hemithioacetal adduct 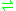 (R)-S-lactoylglutathione
(R)-S-lactoylglutathione
The systematic name of this enzyme class is (R)-S-lactoylglutathione methylglyoxal-lyase (isomerizing glutathione-forming); other names include methylglyoxalase, aldoketomutase, ketone-aldehyde mutase, and (R)-S-lactoylglutathione methylglyoxal-lyase (isomerizing). In some instances, the glutathionyl moiety may be supplied by trypanothione
Trypanothione
Trypanothione is an unusual form of glutathione containing two molecules of glutathione joined by a spermidine linker. It is found in parasitic protozoa such as leishmania and trypanosomes. These protozoal parasites are the cause of leishmaniasis, sleeping sickness and Chagas' disease....
, the analog of glutathione in parasitic protozoa such as the trypanosome
Trypanosoma
Trypanosoma is a genus of kinetoplastids , a monophyletic group of unicellular parasitic flagellate protozoa. The name is derived from the Greek trypano and soma because of their corkscrew-like motion. All trypanosomes are heteroxenous and are transmitted via a vector...
s. The human gene for this enzyme is called GLO1
GLO1
Lactoylglutathione lyase is an enzyme that in humans is encoded by the GLO1 gene. It is a Lactoylglutathione lyase.Recent research demonstrates that GLO1 expression is upregulated in various human malignant tumors including metastatic melanoma....
.
Glyoxalase I derives its name from its catalysis of the first step in the glyoxalase system
Glyoxalase system
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism...
, a critical two-step detoxification system for methylglyoxal
Methylglyoxal
Methylglyoxal, also called pyruvaldehyde or 2-oxopropanal is the aldehyde form of pyruvic acid. It has two carbonyl groups, so it is a dicarbonyl compound. Methylglyoxal is both an aldehyde and a ketone....
. Methylglyoxal is produced naturally as a byproduct of normal biochemistry, but is highly toxic, due to its chemical reactions with protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s, and other cellular components. The second detoxification step, in which (R)-S-lactoylglutathione is split into glutathione and D-lactate, is carried out by glyoxalase II, a hydrolase
Hydrolase
In biochemistry, a hydrolase is an enzyme that catalyzes the hydrolysis of a chemical bond. For example, an enzyme that catalyzed the following reaction is a hydrolase:-Nomenclature:...
. Unusually, these reactions carried out by the glyoxalase system does not oxidize glutathione, which usually acts as a redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
coenzyme. Although aldose reductase
Aldose reductase
Aldose reductase is an NADPH-dependent oxidoreductase that catalyzes the reduction of a variety of aldehydes and carbonyls, including monosaccharides...
can also detoxify methylgloxal, the glyoxalase system is more efficient and seems to be the most important of these pathways. Glyoxalase I is an attractive target for the development of drugs to treat infections by some parasitic protozoa, and cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
. Several inhibitors
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
of glyoxalase I have been identified, such as S-(N-hydroxy-N-methylcarbamoyl)glutathione.
Glyoxalase I is classified as a carbon-sulfur lyase
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
although, strictly speaking, the enzyme does not form or break a carbon-sulfur bond. Rather, the enzyme shifts two hydrogen atoms from one carbon atom of the methylglyoxal to the adjacent carbon atom. In effect, the reaction is an intramolecular redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reaction; one carbon is oxidized whereas the other is reduced. The mechanism proceeds by subtracting and then adding proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s, forming an enediolate intermediate, rather than by transferring hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
s. Unusually for a metalloprotein
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. Indeed, about one quarter to one third of all proteins require metals to...
, this enzyme shows activity with several different metals. Glyoxalase I is also unusual in that it is stereospecific
Stereospecificity
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one of the stereoisomers."Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline...
in the second half of its mechanism, but not in the first half. Structurally, the enzyme is a domain-swapped dimer in many species, although the two subunits have merged into a monomer in yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
, through gene duplication
Gene duplication
Gene duplication is any duplication of a region of DNA that contains a gene; it may occur as an error in homologous recombination, a retrotransposition event, or duplication of an entire chromosome.The second copy of the gene is often free from selective pressure — that is, mutations of it have no...
.
Detoxification of methylglyoxal and other physiological roles
The principal physiological function of glyoxalase I is the detoxification of methylglyoxalMethylglyoxal
Methylglyoxal, also called pyruvaldehyde or 2-oxopropanal is the aldehyde form of pyruvic acid. It has two carbonyl groups, so it is a dicarbonyl compound. Methylglyoxal is both an aldehyde and a ketone....
, a reactive 2-oxoaldehyde that is cytostatic at low concentrations and cytotoxic at millimolar concentrations. Methylglyoxal is a by-product of normal biochemistry that is a carcinogen, a mutagen and can chemically damage several components of the cell, such as proteins and nucleic acids. Methylglyoxal is formed spontaneously from dihydroxyacetone phosphate, enzymatically by triosephosphate isomerase and methylglyoxal synthase, as also in the catabolism of threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
.
To minimize the amount of toxic methylglyoxal and other reactive 2-oxoaldehydes, the glyoxalase system
Glyoxalase system
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism...
has evolved. The methylglyoxal reacts spontaneously with reduced glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
(or its equivalent, trypanothione
Trypanothione
Trypanothione is an unusual form of glutathione containing two molecules of glutathione joined by a spermidine linker. It is found in parasitic protozoa such as leishmania and trypanosomes. These protozoal parasites are the cause of leishmaniasis, sleeping sickness and Chagas' disease....
), forming a hemithioacetal. The glyoxalase system converts such compounds into D-lactate
Lactate
Lactate may refer to:*The act of lactation*The conjugate base of lactic acid...
and restored the glutathione. In this conversion, the two carbonyl carbons of the 2-oxoaldehyde are oxidized and reduced, respectively, the aldehyde being oxidized to a carboxylic acid and the acetal group being reduced to an alcohol. The glyoxalase system evolved very early in life's history and is found nearly universally through life-forms.
The glyoaxalase system consists of two enzymes, glyoxalase I and glyoxalase II. The former enzyme, described here, rearranges the hemithioacetal formed naturally by the attack of glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
on methylglyoxal into the product. Glyoxalase II hydrolyzes the product to re-form the glutathione and produce D-lactate
Lactate
Lactate may refer to:*The act of lactation*The conjugate base of lactic acid...
. Thus, glutathione acts unusually as a coenzyme and is required only in catalytic (i.e., very small) amounts; normally, glutathione acts instead as a redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
couple in oxidation-reduction reactions.
The glyoxalase system has also been suggested to play a role in regulating cell growth and in assembling microtubule
Microtubule
Microtubules are a component of the cytoskeleton. These rope-like polymers of tubulin can grow as long as 25 micrometers and are highly dynamic. The outer diameter of microtubule is about 25 nm. Microtubules are important for maintaining cell structure, providing platforms for intracellular...
s.
Pharmaceutical drug target
Glyoxalase I is a target for the development of pharmaceuticals against bacteria, protozoans (especially Trypanosoma cruziTrypanosoma cruzi
Trypanosoma cruzi is a species of parasitic euglenoid trypanosomes. This species causes the trypanosomiasis diseases in humans and animals in America...
and the Leishmania
Leishmania
Leishmania is a genus of Trypanosomatid protozoa, and is the parasite responsible for the disease leishmaniasis. It is spread through sandflies of the genus Phlebotomus in the Old World, and of the genus Lutzomyia in the New World. Their primary hosts are vertebrates; Leishmania commonly infects...
) and human cancer. Numerous inhibitors have been developed, most of which share the glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
moiety. Among the most tightly binding family of inhibitors to the human enzyme are derivatives of S-(N-aryl-N-hydroxycarbamoyl)glutathione, most notably the p-bromophenyl derivative, which has a dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
of 14 nM. The closest analog of the transition state is believed to be S-(N-hydroxy-N-p-iodophenylcarbamoyl)glutathione; the crystal structure of this compound bound to the human enzyme has been solved to 2 Å resolution (PDB accession code ).
Experiments suggest that methylglyoxal is preferentially toxic to proliferating cells, such as those in cancer.
Unusual enzymatic properties: lack of metal specificity and stereochemistry
Glyoxalase I requires bound metal ions for catalysis. The human enzyme and its counterparts in yeast (Saccharomyces cerevisiaeSaccharomyces cerevisiae
Saccharomyces cerevisiae is a species of yeast. It is perhaps the most useful yeast, having been instrumental to baking and brewing since ancient times. It is believed that it was originally isolated from the skin of grapes...
) and Pseudomonas putida
Pseudomonas putida
Pseudomonas putida is a gram-negative rod-shaped saprotrophic soil bacterium. Based on 16S rRNA analysis, P. putida has been placed in the P. putida group, to which it lends its name....
use divalent zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, Zn2+. By contrast, the prokaryotic versions often use a nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
ion. Interestingly, the glyoxalase I found in eukaryotic trypanosomal parasites such as Leishmania major
Leishmania major
Leishmania major is a species of Leishmania.It is associated with zoonotic cutaneous leishmaniasis.The genome has been sequenced....
and Trypanosoma cruzi
Trypanosoma cruzi
Trypanosoma cruzi is a species of parasitic euglenoid trypanosomes. This species causes the trypanosomiasis diseases in humans and animals in America...
can also use nickel for activity, possibly reflecting an acquisition of their GLO1 gene by horizontal gene transfer
Horizontal gene transfer
Horizontal gene transfer , also lateral gene transfer , is any process in which an organism incorporates genetic material from another organism without being the offspring of that organism...
.
A striking property of glyoxalase I is its lack of specificity for the catalytic metal ion. Most enzymes prefer to bind one particular type of metal, and their catalytic activity depends on having bound that metal. For example, oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s often use a specific metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
ion such as iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, manganese
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
or copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
and will fail to function if their preferred metal ion is replaced, due to differences in the redox potential
Reduction potential
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts , or millivolts...
; thus, the ferrous superoxide dismutase
Superoxide dismutase
Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen...
cannot function if its catalytic iron is replaced by manganese, and vice versa. By contrast, although human glyoxalase I prefers to use divalent zinc, it is able to function with many other divalent metals, including magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
, manganese
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
, cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
, nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and even calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
.; however, the enzyme is inactive with the ferrous cation. Similarly, although the prokaryotic glyoxalase I prefers nickel, it is able to function with cobalt, manganese and cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
; however, the enzyme is inert with bound zinc, due to a change in coordination geometry
Coordination geometry
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics.-Molecules:The coordination geometry of an atom is the geometrical pattern formed by atoms around the central atom....
from octahedral
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
to trigonal bipyramidal. Structural and computational studies have revealed that the metal binds the two carbonyl oxygens of the methylglyoxal moiety at two of its coordination sites, stabilizing the enediolate anion intermediate.
Another unusual property of glyoxalase I is its inconsistent stereospecificity. The first step of its reaction mechanism (the abstraction of the proton from C1 and subsequent protonation of O2) is not sterospecific, and works equally well regardless of the initial chirality at C1 in the hemithioacetal substrate. The resulting enediolate intermediate is achiral, but the second step of the reaction mechanism (the abstraction of a proton from O1 and subsequent protonation of C2) is definitely stereospecific, producing only the (S) form of D-lactoylglutathione. This is believed to result from the two glutamates bound oppositely on the metal ion; either one is able to carry out the first step, but only one is able to carry out the second step. The reason from this asymmetry is not yet fully determined.
Enzyme mechanism
The methylglyoxalMethylglyoxal
Methylglyoxal, also called pyruvaldehyde or 2-oxopropanal is the aldehyde form of pyruvic acid. It has two carbonyl groups, so it is a dicarbonyl compound. Methylglyoxal is both an aldehyde and a ketone....
molecule consists of two carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups flanked by a hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom and a methyl group. In the discussion below, these two carbonyl carbons will be denoted as C1 and C2, respectively. In both the hemithioacetal substrate and the (R)-S-lactoylglutathione product, the glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
moiety is bonded to the C1 carbonyl group.
The basic mechanism of glyoxalase I is as follows. The substrate hemithioacetal is formed when a molecule of glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
— probably in its reactive thiolate form — attacks the C1 carbonyl of methylglyoxal or a related compound, rendering that carbon tetravalent. This reaction occurs spontaneously in the cell, without the involvement of the enzyme. This hemithioacetalis then bound by the enzyme, which shifts a hydrogen from C1 to C2. The C2 carbonyl is reduced to a tetravalent alcohol form by the addition of two protons, whereas the C1 carbonyl is restored by losing a hydrogen while retaining its bond to the glutathione moiety.
Proton vs. hydride transfer
Glyoxalase I was originally believed to operate by the transfer of a hydrideHydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
, which is a proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
surrounded by two electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s (H–). In this, it was thought to resemble the classic Cannizzaro reaction
Cannizzaro reaction
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position...
mechanism, in which the attack of a hydroxylate on an aldehyde renders it into a tetravalent alcohol anion; this anion donates its hydrogens to a second aldehyde, forming a carboxylic acid and an alcohol. (In effect, two identical aldehydes reduce and oxidize each other, leaving the net oxidation state the same.)
In glyoxalase I, such a hydride-transfer mechanism would work as follows. The attack of the glutathione would leave a charged O– and the aldehyde hydrogen bound to C1. If the carbonyl oxygen of C2 can secure a hydrogen from an obliging acidic sidechain of the enzyme, forming an alcohol, then the hydrogen of C1 might simultaneously slide over with its electrons onto C2 (the hydride transfer). At the same time, the extra electron on the oxygen of C1 could reform the double bond of the carbonyl, thus giving the final product.
An alternative (and ultimately correct) mechanism using proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
(H+) transfer was put forward in the 1970s. In this mechanism, a basic sidechain of the enzyme abstracts the aldehyde proton from C1; at the same time, the a proton is added to the oxygen of C2, thus forming a enediol. The ene means that a double bond has formed between C2 and C1, from the electrons left behind by the abstraction of the aldehyde proton; the diol refers to the fact that two alcohols have been made of the initial two carbonyl groups. In this mechanism, the intermediate forms the product by adding another proton to C2.
It was expected that solvent protons would contribute to forming the product from the enediol intermediate of the proton-transfer mechanism and when such contributions were not observed in tritiated
Tritium
Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
water, 3H1O, the hydride-transfer mechanism was favored. However, an alternate hypothesis — that the enzyme active site was deeply buried away from water — could not be ruled out and ultimately proved to be correct. The first indications came when ever-increasing temperatures showed ever-increasing incorporation of tritium, which is consistent with proton transfer and unexpected by hydride transfer. The clinching evidence can with studies of the hydrogen-deuterium isotope effect
Isotope effect
Isotope effect can refer to:* Kinetic isotope effect* Magnetic isotope effect* Superconductive transition temperature varying by isotope atomic weight; See BCS theory#Successes of the BCS theory...
on substrates fluorinated
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
on the methyl group and deuterated on the aldehyde. The fluoride is a good leaving group; the hydride-transfer mechanism predicts less fluoride ion elimination with the deuterated sample, whereas the proton-transfer mechanism predicts more. Experiments on three types of glyoxalase I (yeast, rat and mouse forms) supported the proton-transfer mechanism in every case. This mechanism was finally observed in crystal structures of glyoxalase I.
Atomic-resolution mechanism
A computational study, combined with the available experimental data, suggests the following atomic-resolution mechanism for glyoxalase I. In the active site, the catalytic metal adopts an octahedral coordination geometry and, in the absence of substrate, binds two waters, two opposite glutamates, a histidineHistidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
and one other sidechain, usually another histidine or glutamates. When the substrate enters the active site, the two waters are shed and the two carbonyl oxygens of the substrate are bound directly to the metal ion. The two opposing glutamates add and subtract protons from C1 and C2 and their respective oxygens, O1 and O2. The first half of the reaction transfers a proton from C1 to O2, whereas the second half transfers a proton from O1 to C2. The former reaction may be carried out by either of the opposing glutamates, depending on the initial chirality of C1 in the hemithioacetal substrate; however, the second half is stereospecific and is carried out by only one of the opposing glutamates.
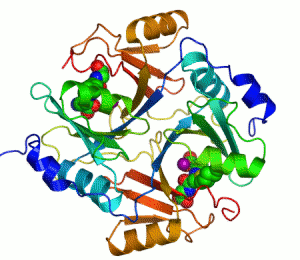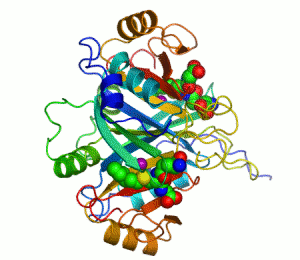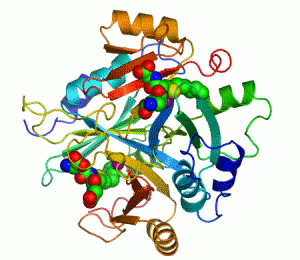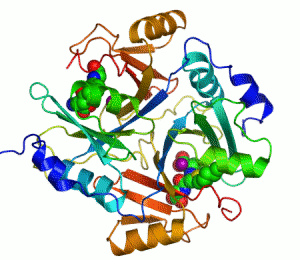
Structure
Several structuresTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
of glyoxalase I have been solved. Four structures of the human form have been published, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , and . Five structures of the Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
form have been published, with accession codes , , , , and . Finally, one structure of the trypanothione-specific version from Leishmania major
Leishmania major
Leishmania major is a species of Leishmania.It is associated with zoonotic cutaneous leishmaniasis.The genome has been sequenced....
has been solved, . In all these cases, the quaternary structure
Quaternary structure
In biochemistry, quaternary structure is the arrangement of multiple folded protein or coiling protein molecules in a multi-subunit complex.-Description and examples:...
of the biological unit is a domain-swapped dimer, in which the active site and the 8-stranded beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
is formed from both subunits. However, in yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
such as Saccharomyces cerevisiae
Saccharomyces cerevisiae
Saccharomyces cerevisiae is a species of yeast. It is perhaps the most useful yeast, having been instrumental to baking and brewing since ancient times. It is believed that it was originally isolated from the skin of grapes...
, the two subunits have fused into a single monomer of double size, through gene duplication
Gene duplication
Gene duplication is any duplication of a region of DNA that contains a gene; it may occur as an error in homologous recombination, a retrotransposition event, or duplication of an entire chromosome.The second copy of the gene is often free from selective pressure — that is, mutations of it have no...
. Each half of the structural dimer is a sandwich of 3-4 alpha helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
on both sides of an 8-stranded antiparallel beta sheet; the dimer interface is largely composed of the face-to-face meeting of the two beta sheets.
The tertiary and quaternary structures of glyoxalase I is similar to those of several other types of proteins. For example, glyoxalase I resembles several proteins that allow bacteria to resist antibiotics such as fosfomycin
Fosfomycin
Fosfomycin is a broad-spectrum antibiotic produced by certain Streptomyces species.-Uses:...
, bleomycin
Bleomycin
Bleomycin is a glycopeptide antibiotic produced by the bacterium Streptomyces verticillus. Bleomycin refers to a family of structurally related compounds. When used as an anticancer agent, the chemotherapeutical forms are primarily bleomycin A2 and B2. It works by causing breaks in DNA...
and mitomycin
Mitomycin
The mitomycins are a family of aziridine-containing natural products isolated from Streptomyces caespitosus or Streptomyces lavendulae. One of these compounds, mitomycin C, finds use as a chemotherapeutic agent by virtue of its antitumour antibiotic activity. It is given intravenously to treat...
. Likewise, the unrelated enzymes methylmalonyl-CoA epimerase, 3-demethylubiquinone-9 3-O-methyltransferase
3-demethylubiquinone-9 3-O-methyltransferase
In enzymology, a 3-demethylubiquinone-9 3-O-methyltransferase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are S-adenosyl methionine and 3-demethylubiquinone-9, whereas its two products are S-adenosylhomocysteine and ubiquinone-9.This enzyme belongs to...
and numerous dioxygenase
Dioxygenase
In molecular biology, a dioxygenase is an enzyme which catalyses the incorporation of both atoms of molecular oxygen into substrates using a variety of reaction mechanisms. Cleavage of aromatic rings is one of the most important functions of dioxygenases, which play key roles in the degradation of...
s such as biphenyl-2,3-diol 1,2-dioxygenase
Biphenyl-2,3-diol 1,2-dioxygenase
In enzymology, a biphenyl-2,3-diol 1,2-dioxygenase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are biphenyl-2,3-diol and O2, whereas its two products are 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate and H2O....
, catechol 2,3-dioxygenase, 3,4-dihydroxyphenylacetate 2,3-dioxygenase
3,4-dihydroxyphenylacetate 2,3-dioxygenase
In enzymology, a 3,4-dihydroxyphenylacetate 2,3-dioxygenase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are 3,4-dihydroxyphenylacetate and O2, whereas its product is 2-hydroxy-5-carboxymethylmuconate semialdehyde....
and 4-hydroxyphenylpyruvate dioxygenase
4-Hydroxyphenylpyruvate dioxygenase
4-Hydroxyphenylpyruvate dioxygenase HPPD is an Fe-containing enzyme, that catalyzes the second reaction in the catabolism of tyrosine - the conversion of 4-hydroxyphenylpyruvate to homogentisate.-Function:...
all resemble glyoxalase I in structure. Finally, many proteins of unknown or uncertain function likewise resemble glyoxalase I, such as At5g48480 from the plant, Arabidopsis thaliana
Arabidopsis thaliana
Arabidopsis thaliana is a small flowering plant native to Europe, Asia, and northwestern Africa. A spring annual with a relatively short life cycle, arabidopsis is popular as a model organism in plant biology and genetics...
.

